Continuing Education Examination available at http://www.cdc.gov/mmwr/cme/conted.html.
Early Release / Vol. 62 June 14, 2013
U.S. Selected Practice Recommendations for
Contraceptive Use, 2013
Adapted from the World Health Organization Selected Practice
Recommendations for Contraceptive Use, 2nd Edition
U.S. Department of Health and Human Services
Centers for Disease Control and Prevention
Morbidity and Mortality Weekly Report

Early Release
CONTENTS (Continued)
Disclosure of Relationship
CDC, our planners, and our content experts wish to disclose
they have no financial interests or other relationships with
the manufacturers of commercial products, suppliers of com-
mercial services, or commercial supporters. Planners have
reviewed content to ensure there is no bias. This document will
not include any discussion of the unlabeled use of a product
or a product under investigational use, with the exception
that some of the recommendations in this document might
be inconsistent with package labeling. CDC does not accept
commercial support.
Front cover photos, left to right: intrauterine device, oral contraceptive pills, diaphragm, syringe for injectable contraceptives, male condom, transdermal
contraceptive patch, etonogestrel implant, vaginal ring.
The MMWR series of publications is published by the Office of Surveillance, Epidemiology, and Laboratory Services, Centers for Disease Control and Prevention (CDC),
U.S. Department of Health and Human Services, Atlanta, GA 30333.
Suggested Citation: Centers for Disease Control and Prevention. [Title]. MMWR 2013;62(No. RR-#):[inclusive page numbers].
Centers for Disease Control and Prevention
Thomas R. Frieden, MD, MPH, Director
Harold W. Jaffe, MD, MA, Associate Director for Science
James W. Stephens, PhD, Director, Office of Science Quality
Denise M. Cardo, MD, Acting Deputy Director for Surveillance, Epidemiology, and Laboratory Services
Stephanie Zaza, MD, MPH, Director, Epidemiology and Analysis Program Office
MMWR Editorial and Production Staff
Ronald L. Moolenaar, MD, MPH, Editor, MMWR Series
Christine G. Casey, MD, Deputy Editor, MMWR Series
Teresa F. Rutledge, Managing Editor, MMWR Series
David C. Johnson, Lead Technical Writer-Editor
Catherine B. Lansdowne, MS, Project Editor
Martha F. Boyd, Lead Visual Information Specialist
Maureen A. Leahy, Julia C. Martinroe,
Stephen R. Spriggs, Terraye M. Starr
Visual Information Specialists
Quang M. Doan, MBA, Phyllis H. King
Information Technology Specialists
MMWR Editorial Board
William L. Roper, MD, MPH, Chapel Hill, NC, Chairman
Matthew L. Boulton, MD, MPH, Ann Arbor, MI
Virginia A. Caine, MD, Indianapolis, IN
Barbara A. Ellis, PhD, MS, Atlanta, GA
Jonathan E. Fielding, MD, MPH, MBA, Los Angeles, CA
David W. Fleming, MD, Seattle, WA
William E. Halperin, MD, DrPH, MPH, Newark, NJ
King K. Holmes, MD, PhD, Seattle, WA
Timothy F. Jones, MD, Nashville, TN
Rima F. Khabbaz, MD, Atlanta, GA
Dennis G. Maki, MD, Madison, WI
Patricia Quinlisk, MD, MPH, Des Moines, IA
Patrick L. Remington, MD, MPH, Madison, WI
John V. Rullan, MD, MPH, San Juan, PR
William Schaffner, MD, Nashville, TN
CONTENTS
Introduction ............................................................................................................1
Methods
....................................................................................................................2
How To Use This Document
............................................................................... 3
Summary of Changes from WHO SPR
............................................................ 4
Contraceptive Method Choice
.........................................................................4
Maintaining Updated Guidance
......................................................................4
How To Be Reasonably Certain that a Woman Is Not Pregnant
............ 5
Intrauterine Contraception
................................................................................7
Implants
................................................................................................................. 14
Injectables
............................................................................................................. 17
Combined Hormonal Contraceptives
......................................................... 22
Progestin-Only Pills
............................................................................................ 29
Standard Days Method
..................................................................................... 33
Emergency Contraception
.............................................................................. 34
Female Sterilization
........................................................................................... 35
Male Sterilization
................................................................................................ 36
When Women Can Stop Using Contraceptives
....................................... 37
Conclusion
............................................................................................................ 37
Acknowledgment
............................................................................................... 38
References
............................................................................................................. 38
Appendix A: Summary Chart of U.S. Medical Eligibility Criteria for
Contraceptive Use, 2010
.................................................................................. 47
Appendix B: When To Start Using Specific Contraceptive
Methods
.............................................................................................................. 55
Appendix C: Examinations and Tests Needed Before Initiation of
Contraceptive Methods
................................................................................. 56
Appendix D: Routine Follow-Up After Contraceptive Initiation
........ 57
Appendix E: Management of Women with Bleeding Irregularities
While Using Contraception
.......................................................................... 58
Appendix F: Management of the IUD when a Cu-IUD or an LNG-IUD
User Is Found To Have Pelvic Inflammatory Disease
........................... 59

Early Release
MMWR / June 14, 2013 / Vol. 62 1
U.S. Selected Practice Recommendations for Contraceptive Use, 2013
Adapted from the World Health Organization Selected Practice
Recommendations for Contraceptive Use, 2nd Edition
Prepared by
Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion
Summary
The U. S. Selected Practice Recommendations for Contraceptive Use 2013 (U.S. SPR), comprises recommendations that
address a select group of common, yet sometimes controversial or complex, issues regarding initiation and use of specific contraceptive
methods. These recommendations are a companion document to the previously published CDC recommendations U.S. Medical
Eligibility Criteria for Contraceptive Use, 2010 (U.S. MEC). U.S. MEC describes who can use various methods of contraception,
whereas this report describes how contraceptive methods can be used. CDC based these U.S. SPR guidelines on the global family
planning guidance provided by the World Health Organization (WHO). Although many of the recommendations are the same
as those provided by WHO, they have been adapted to be more specific to U.S. practices or have been modified because of new
evidence. In addition, four new topics are addressed, including the effectiveness of female sterilization, extended use of combined
hormonal methods and bleeding problems, starting regular contraception after use of emergency contraception, and determining
when contraception is no longer needed. The recommendations in this report are intended to serve as a source of clinical guidance
for health-care providers; health-care providers should always consider the individual clinical circumstances of each person seeking
family planning services. This report is not intended to be a substitute for professional medical advice for individual patients.
Persons should seek advice from their health-care providers when considering family planning options.
Introduction
Unintended pregnancy rates remain high in the United
States; approximately 50% of all pregnancies are unintended,
with higher proportions among adolescent and young women,
women who are racial/ethnic minorities, and women with lower
levels of education and income (1). Unintended pregnancies
increase the risk for poor maternal and infant outcomes (2)
and in 2002, resulted in $5 billion in direct medical costs in the
United States (3). Approximately half of unintended pregnancies
are among women who were not using contraception at the
time they became pregnant; the other half are among women
who became pregnant despite reported use of contraception
(4). Therefore, strategies to prevent unintended pregnancy
include assisting women at risk for unintended pregnancy and
their partners with choosing appropriate contraceptive methods
and helping women use methods correctly and consistently
to prevent pregnancy. In 2010, CDC first adapted global
guidance from the World Health Organization (WHO) to
help health-care providers counsel women, men, and couples
about contraceptive method choice. The U.S. Medical Eligibility
Criteria for Contraceptive Use, 2010 (U.S. MEC), focuses on who
can safely use specific methods of contraception and provides
recommendations for the safety of contraceptive methods for
women with various medical conditions (e.g., hypertension and
diabetes) and characteristics (e.g., age, parity, and smoking status)
(Appendix A) (5). The recommendations in this new guide, U.S.
Selected Practice Recommendations for Contraceptive Use, 2013
(U.S. SPR), focuses on how contraceptive methods can be used
and provides recommendations on optimal use of contraceptive
methods for persons of all ages, including adolescents.
During the past 15 years, CDC has contributed to the
development and updating of the WHO global family planning
guidance. CDC has supported WHO by coordinating the
identification, critical appraisal, and synthesis of the scientific
evidence on which the WHO guidance is based. In 2002,
WHO published the first edition of the Selected Practice
Recommendations for Contraceptive Use (WHO SPR), which
presented evidence-based global guidance on how to use
contraceptive methods safely and effectively once they are
deemed to be medically appropriate. Since then, WHO has
regularly updated its guidance on the basis of new evidence,
and the document is now in its second edition (6), with an
additional update in 2008 (7). The WHO global guidance is
not intended for use directly by health-care providers; rather,
WHO intends for the guidance to be used by local or national
policy makers, family planning program managers, and the
The material in this report originated in the National Center for
Chronic Disease Prevention and Health Promotion, Ursula Bauer,
PhD, Director; Division of Reproductive Health, Wanda Barfield,
MD, Director.
Corresponding preparer: Kathryn M. Curtis, PhD, Division of
Reproductive Health. Telephone: 770-488-5200; E-mail: [email protected].
Early Release
2 MMWR / June 14, 2013 / Vol. 62
scientific community as a reference when they develop family
planning guidance at the country or program level (6). For
example, the United Kingdom adapted WHO SPR and in
2002 published the U.K. Selected Practice Recommendations
for Contraceptive Use for use by U.K. health-care providers (8).
CDC initiated a formal adaptation process to create U.S.
SPR, using both the second edition of WHO SPR (6) and the
2008 update (7) as the basis for the U.S. version. Although
much of the guidance is the same as the WHO guidance,
the recommendations are specific to U.S. family planning
practice. In addition, guidance on contraceptive methods not
available in the United States has been removed, and four
new topics for guidance have been added (the effectiveness
of female sterilization, extended use of combined hormonal
methods and bleeding problems, starting regular contraception
after use of emergency contraception, and determining when
contraception is no longer needed). This document contains
recommendations for health-care providers for the safe and
effective use of contraceptive methods and addresses provision of
contraceptive methods and management of side effects and other
problems with contraceptive method use. Although the term
woman is used throughout this report, these recommendations
refer to all females of reproductive age, including adolescents.
Adolescents are identified throughout this document as a special
population that might benefit from more frequent follow-up.
These recommendations are meant to serve as a source of
clinical guidance for health-care providers; health-care providers
should always consider the individual clinical circumstances
of each person seeking family planning services. This report is
not intended to be a substitute for professional medical advice
for individual patients; persons should seek advice from their
health-care providers when considering family planning options.
Methods
CDC initiated a process to adapt WHO SPR for the
United States. This adaptation process included four steps:
1) determining the scope of and process for the adaptation,
including an October 2010 meeting in which individual
feedback was solicited from a small group of partners and
experts; 2) preparing the systematic reviews of the evidence
during October 2010–September 2011 to be used for the
adaptation, including peer review; 3) convening a larger
meeting of experts in October 2011 to examine the evidence
and receive input on the recommendations; and 4) finalizing
recommendations by CDC.
During October 21–22, 2010, CDC convened a meeting of 10
partners and U.S. family planning experts in Atlanta, Georgia, to
discuss the scope of and process for a U.S. adaptation of WHO
SPR. A list of participants is provided at the end of this report.
CDC identified the specific WHO recommendations that might
benefit from modification for the United States. Criteria used to
modify the WHO recommendations included the availability of
new scientific evidence or the context in which family planning
services are provided in the United States. CDC also identified
several WHO recommendations that needed additional specificity
to be useful for U.S. health-care providers, as well as the need for
additional recommendations not currently included in WHO
SPR. In addition, the meeting members discussed removing
recommendations that provide information about contraceptive
methods that are not available in the United States.
Representatives from CDC and WHO conducted systematic
reviews of the scientific evidence for each of the WHO
recommendations being considered for adaptation and for each
new topic being considered for addition to the guidance. The
purpose of these systematic reviews was to identify evidence
related to the common clinical challenges associated with the
recommendations. When no direct evidence was available,
indirect evidence and theoretical issues were considered. Standard
guidelines were followed for reporting systematic reviews (9,10),
and strength and quality of the evidence were graded using the
system of the U.S. Preventive Services Task Force (11). Each
complete systematic review was peer reviewed by two or three
experts before its use in the adaptation process. Peer reviewers,
who were identified from the list of persons scheduled to
participate in the October 2011 meeting, were asked to comment
on the search strategy, list of articles included in the reviews, and
the summary of findings. The systematic reviews were finalized
and provided to participants before the October 2011 meeting
and were published in May 2013 (12–30).
During October 4–7, 2011, CDC convened a meeting in
Atlanta, Georgia, of 36 experts who were invited to assist in
guideline development and provide their perspective on the
scientific evidence presented and the discussions on potential
recommendations that followed. The group included obstetrician/
gynecologists, pediatricians, family physicians, nurse-midwives,
nurse practitioners, epidemiologists, and others with research and
clinical practice expertise in contraceptive safety, effectiveness, and
management. All participants received all of the systematic reviews
before the meeting. During the meeting, the evidence from the
systematic review for each topic was presented, and participants
discussed the evidence and the translation of the scientific evidence
into recommendations that would meet the needs of U.S. health-
care providers. In particular, participants discussed whether and
how the U.S. context might be different from the global context
and whether these differences suggested any need for modifications
to the global guidance. CDC gathered the input from the experts
during the meeting and finalized the recommendations in this
Early Release
MMWR / June 14, 2013 / Vol. 62 3
report. The document was peer reviewed by meeting participants,
who were asked to comment on specific issues that were raised
during the meeting. Feedback also was received from an external
review panel, composed of health-care providers who had not
participated in the adaptation meetings. These providers were
asked to provide comments on the accuracy, feasibility, and clarity
of the recommendations, as well as to provide other comments.
Areas of research that need additional investigation also were
considered during the meeting (31).
How To Use This Document
The recommendations in this report are intended to
help health-care providers address issues related to use of
contraceptives, such as how to help a woman initiate use of a
contraceptive method, which examinations and tests are needed
before initiating use of a contraceptive method, what regular
follow-up is needed, and how to address problems that often
arise during use, including missed pills and side effects such as
unscheduled bleeding. Each recommendation addresses what
a woman or health-care provider can do in specific situations.
For situations in which certain groups of women might be
medically ineligible to follow the recommendations, comments
and reference to U.S. MEC are provided (5). The full U.S.
MEC recommendations and the evidence supporting those
recommendations were published in 2010 (5).
The information in this document is organized by
contraceptive method, and the methods generally are presented
in order of effectiveness, from highest to lowest. However, the
recommendations are not intended to provide guidance on
every aspect of provision and management of contraceptive
method use. Instead, they use the best available evidence
to address specific issues regarding common, yet sometimes
complex, clinical issues. Each contraceptive method section
generally includes information about initiation of the method,
regular follow-up, and management of problems with use (e.g.,
usage errors and side effects). Each section first provides the
recommendation and then includes a comments and evidence
section, which includes comments about the recommendations
and a brief summary of the scientific evidence on which the
recommendation is based.
Recommendations in this document are provided for
permanent methods of contraception, such as vasectomy
and female sterilization, as well as for reversible methods of
contraception, including the copper-containing intrauterine
device (Cu-IUD); levonorgestrel-releasing IUD (LNG-IUD);
the etonogestrel implant; progestin-only injectables; progestin-
only pills (POPs); combined hormonal contraceptive methods
that contain both estrogen and a progestin, including combined
oral contraceptives (COCs), a transdermal contraceptive patch,
and a vaginal contraceptive ring; and the standard days method
(SDM). Recommendations also are provided for emergency
use of the Cu-IUD and emergency contraceptive pills (ECPs).
For each contraceptive method, recommendations are provided
on the timing for initiation of the method and indications for
when and for how long additional contraception, or a back-up
method, is needed. Many of these recommendations include
guidance that a woman can start a contraceptive method at any
time during her menstrual cycle if it is reasonably certain that
the woman is not pregnant. Guidance for health-care providers
on how to be reasonably certain that a woman is not pregnant
is provided.
For each contraceptive method, recommendations include the
examinations and tests needed before initiation of the method.
These recommendations apply to persons who are presumed to
be healthy. Those with known medical problems or other special
conditions might need additional examinations or tests before
being determined to be appropriate candidates for a particular
method of contraception. U.S. MEC might be useful in such
circumstances (5). Most women need no or very few examinations
or tests before initiating a contraceptive method. The following
classification system was developed by WHO and adopted by
CDC to categorize the applicability of the various examinations
or tests before initiation of contraceptive methods (6):
Class A:These tests and examinations are essential and
mandatory in all circumstances for safe and effective use of
the contraceptive method.
Class B: These tests and examinations contribute substantially
to safe and effective use, although implementation can be
considered within the public health context, service context, or
both. The risk for not performing an examination or test should
be balanced against the benefits of making the contraceptive
method available.
Class C: These tests and examinations do not contribute
substantially to safe and effective use of the contraceptive method.
These classifications focus on the relation of the examinations
or tests to safe initiation of a contraceptive method. They
are not intended to address the appropriateness of these
examinations or tests in other circumstances. For example,
some of the examinations or tests that are not deemed necessary
for safe and effective contraceptive use might be appropriate
for good preventive health care or for diagnosing or assessing
suspected medical conditions. Systematic reviews were
conducted for several different types of examinations and tests
to assess whether a screening test was associated with safe use
of contraceptive methods.Because no single convention exists
for screening panels for certain diseases, including diabetes,
Early Release
4 MMWR / June 14, 2013 / Vol. 62
lipid disorders, and liver diseases, the search strategies included
broad terms for the tests and diseases of interest.
Summary charts and clinical algorithms that summarize
the guidance for the various contraceptive methods have been
developed for many of the recommendations, including when
to start using specific contraceptive methods (Appendix B),
examinations and tests needed before initiating the various
contraceptive methods (Appendix C), routine follow-up after
initiating contraception (Appendix D), management of bleeding
irregularities (Appendix E), and management of IUDs when
users are found to have pelvic inflammatory disease (PID)
(Appendix F). These summaries might be helpful to health-care
providers when managing family planning patients. Additional
tools are available on the U.S. SPR website (http://www.cdc.
gov/reproductivehealth/UnintendedPregnancy/USSPR.htm).
Summary of Changes from WHO SPR
Much of the guidance in U.S. SPR is the same or very similar
to the WHO SPR guidance. U.S. SPR includes new guidance
on the use of the combined contraceptive patch and vaginal
ring, as well as recommendations for four new topics:
• howtostartregularcontraceptionaftertakingECPs
• management of bleeding irregularitiesamongwomen
using extended or continuous combined hormonal
contraceptives (including pills, the patch, and the ring)
• whenawomancanrelyonfemalesterilizationforcontraception
• whenawomancanstopusingcontraceptivesandnotbe
at risk for unintended pregnancy
Adaptations to the WHO SPR recommendations include
1) changes to the length of the grace period for depot
medroxyprogesterone acetate (DMPA) reinjection, 2) differences
in some of the examinations and tests recommended before
contraceptive method initiation, 3) differences in some of the
recommendations for management of bleeding irregularities
because of new data and drug availability in the United States,
and 4) a modified missed pill algorithm to respond to concerns
of the CDC expert group and other reviewers that simplified
algorithms are preferable.
Contraceptive Method Choice
Many elements need to be considered individually by a
woman, man, or couple when choosing the most appropriate
contraceptive method. Some of these elements include
safety, effectiveness, availability (including accessibility and
affordability), and acceptability.
Contraceptive method effectiveness is critically important
in minimizing the risk for unintended pregnancy, particularly
among women for whom an unintended pregnancy would
pose additional health risks. The effectiveness of contraceptive
methods depends both on the inherent effectiveness of the
method itself and on how consistently and correctly it is used
(Table 1). Both consistent and correct use can vary greatly
with characteristics such as age, income, desire to prevent
or delay pregnancy, and culture. Methods that depend on
consistent and correct use by clients have a wide range of
effectiveness between typical and perfect users. IUDs and
implants are considered long-acting, reversible contraception
(LARC); these methods are highly effective because they do not
depend on regular compliance from the user. LARC methods
are appropriate for most women, including adolescents and
nulliparous women. All women should be counseled about
the full range and effectiveness of contraceptive options for
which they are medically eligible so that they can identify the
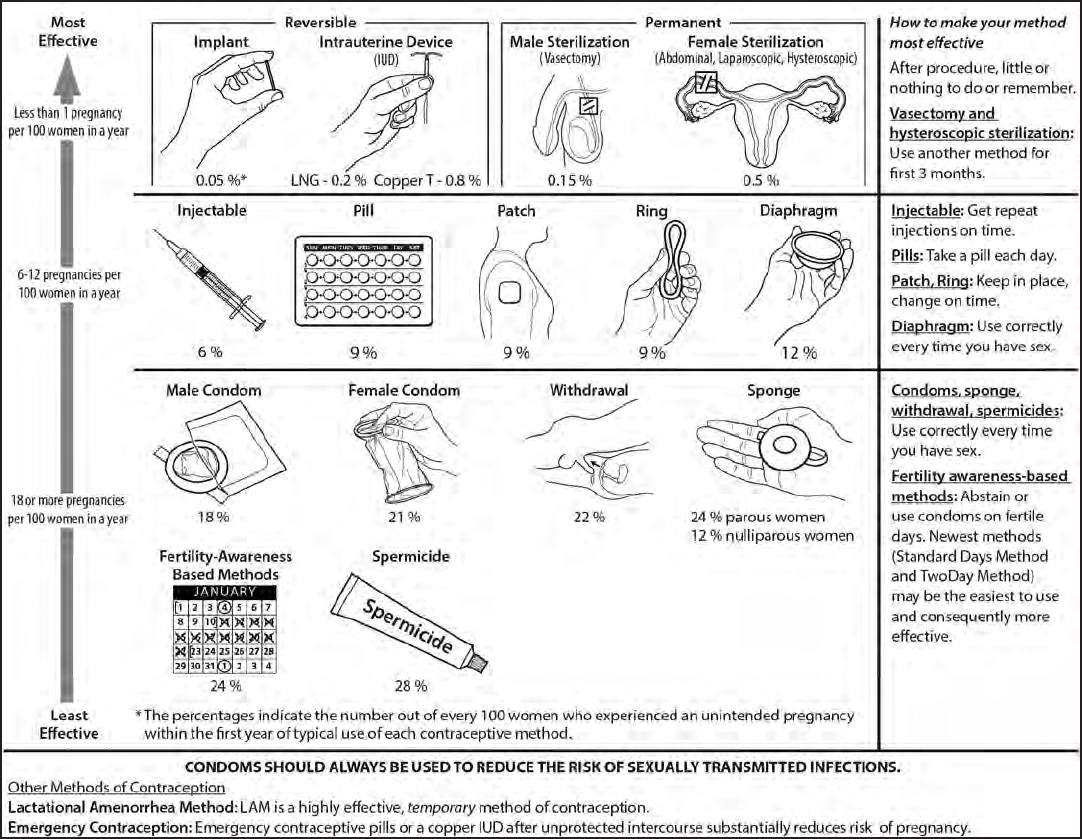
optimal method (Figure 1).
In choosing a method of contraception, the risk for human
immunodeficiency virus (HIV) infection and other sexually
transmitted diseases (STDs) also should be considered.
Although hormonal contraceptives and IUDs are highly
effective at preventing pregnancy, they do not protect against
STDs and HIV. Consistent and correct use of the male latex
condom reduces the risk for HIV infection and other STDs,
including chlamydial infection, gonorrhea, and trichomoniasis
(32). On the basis of a limited number of clinical studies, when
a male condom cannot be used properly to prevent infection,
a female condom should be considered (32). All patients,
regardless of contraceptive choice, should be counseled about
the use of condoms and the risk for STDs, including HIV
infection (32). Additional information about prevention
and treatment of STDs is available from the CDC Sexually
Transmitted Diseases Treatment Guidelines (32).
Maintaining Updated Guidance
As with any evidence-based guidance document, a key
challenge is keeping the recommendations up to date as new
scientific evidence becomes available. Working with WHO,
CDC uses the continuous identification of research evidence
(CIRE) system to ensure that WHO and CDC guidance is
based on the best available evidence and that a mechanism
is in place to update guidance when new evidence becomes
available (33). CDC will continue to work with WHO to
identify and assess all new relevant evidence and determine
whether changes in the recommendations are warranted. In

Early Release
MMWR / June 14, 2013 / Vol. 62 5
most cases, U.S. SPR will follow any updates in the WHO
guidance, which typically occurs every 3–4 years (or sooner
if warranted by new data). In addition, CDC will review any
interim WHO updates for their application in the United
States. CDC also will identify and assess any new literature
for the recommendations that are not included in the WHO
guidance and will completely review U.S. SPR every 3–4
years. Updates to the guidance can be found on the U.S.
SPR website (http://www.cdc.gov/reproductivehealth/
UnintendedPregnancy/USSPR.htm).
How To Be Reasonably Certain that a
Woman Is Not Pregnant
In most cases, a detailed history provides the most accurate
assessment of pregnancy risk in a woman who is about to start
using a contraceptive method. Several criteria for assessing
pregnancy risk are listed in the recommendation that follows.
These criteria are highly accurate (i.e., a negative predictive
value of 99%–100%) in ruling out pregnancy among women
who are not pregnant (34–37). Therefore, CDC recommends
that health-care providers use these criteria to assess pregnancy
TABLE 1. Percentage of women experiencing an unintended pregnancy during the first year of typical use and the first year of perfect use of
contraception and the percentage continuing use at the end of the first year — United States
Method
% of women experiencing an unintended pregnancy
within the first year of use
% of women continuing use at 1 year
§
Typical use* Perfect use
†
No method
¶
85 85 —
Spermicides** 28 18 42
Fertility awareness–based methods
††
24 — 47
Standard days method — 5 —
Two day method — 4 —
Ovulation method — 3 —
Symptothermal method — 0.4 —
Withdrawal 22 4 46
Sponge
Parous women 24 20 36
Nulliparous women 12 9 —
Condom
§§
Female 21 5 41
Male 18 2 43
Diaphragm*** 12 6 57
Combined pill and progestin-only pill 9 0.3 67
Evra patch 9 0.3 67
NuvaRing 9 0.3 67
Depo-Provera 6 0.2 56
Intrauterine devices
Paragard (copper containing) 0.8 0.6 78
Mirena (levenorgestrel releasing) 0.2 0.2 80
Implanon 0.05 0.05 84
Female sterilization 0.5 0.5 100
Male sterilization 0.15 0.10 100
Lactational amenorrhea method
†††
— — —
Source: Adapted from Trussell J. Contraceptive failure in the United States. Contraception 2011;83:397–404.
* Among typical couples who initiate use of a method (not necessarily for the first time), the percentage who experience an accidental pregnancy during the first
year if they do not stop use for any other reason. Estimates of the probability of pregnancy during the first year of typical use for spermicides and the diaphragm
are taken from the 1995 National Survey of Family Growth (NSFG) corrected for underreporting of abortion; estimates for fertility awareness-based methods,
withdrawal, the male condom, the pill and Depo-Provera are taken from the 1995 and 2002 NSFG corrected for underreporting of abortion.
†
Among couples who initiate use of a method (not necessarily for the first time) and who use it perfectly (both consistently and correctly), the percentage who
experience an accidental pregnancy during the first year if they do not stop use for any other reason.
§
Among couples attempting to avoid pregnancy, the percentage who continues to use a method for 1 year.
¶
The percentage becoming pregnant in the second and third columns are based on data from populations where contraception is not used and from women who
cease using contraception to become pregnant. Among such populations, approximately 89% become pregnant within 1 year. This estimate was lowered slightly
(to 85%) to represent the percentage who would become pregnant within 1 year among women not relying on reversible methods of contraception if they
abandoned contraception altogether.
** Foams, creams, gels, vaginal suppositories, and vaginal film.
††
The ovulation and two day methods are based on evaluation of cervical mucus. The standard days method avoids intercourse on cycle days 8–19. The symptothermal
method is a double-check method based on evaluation of cervical mucus to determine the first fertile day and evaluation of cervical mucus and temperature to
determine the last fertile day.
§§
Without spermicides.
*** With spermicidal cream or jelly.
†††
This is a highly effective, temporary method of contraception. However, to maintain in effective protection against pregnancy, another method of contraception must
be used as soon as menstruation resumes, the frequency of duration of breastfeeds is reduced, bottle feeds are introduced, or the baby reaches age 6 months.
Early Release
6 MMWR / June 14, 2013 / Vol. 62
status in a woman who is about to start using contraceptives
(Box 1). If a woman meets one of these criteria (and therefore
the health-care provider can be reasonably certain that she is
not pregnant), a urine pregnancy test might be considered
in addition to these criteria (based on clinical judgment),
bearing in mind the limitations of the accuracy of pregnancy
testing. If a woman does not meet any of these criteria, then
the health-care provider cannot be reasonably certain that she
is not pregnant, even with a negative pregnancy test. Routine
pregnancy testing for every woman is not necessary.
On the basis of clinical judgment, health-care providers
might consider the addition of a urine pregnancy test; however,
they should be aware of the limitations, including accuracy
of the test relative to the time of last sexual intercourse,
recent delivery, or spontaneous or induced abortion. Routine
pregnancy testing for every woman is not necessary. If a woman
has had recent (i.e., within the last 5 days) unprotected sexual
intercourse, consider offering emergency contraception (either
a Cu-IUD or ECPs), if pregnancy is not desired.
Comments and Evidence Summary. The criteria for
determining whether a woman is pregnant depend on the
assurance that she has not ovulated within a certain amount of
time after her last menses, spontaneous or induced abortion, or
delivery. Among menstruating women, the timing of ovulation
can vary widely. During an average 28-day cycle, ovulation
generally occurs during days 9–20 (38). In addition, the
FIGURE 1. Effectiveness of family planning methods
Sources: Adapted from World Health Organization (WHO) Department of Reproductive Health and Research, Johns Hopkins Bloomberg School of Public Health/
Center for Communication Programs (CCP). Knowledge for health project. Family planning: a global handbook for providers (2011 update). Baltimore, MD; Geneva,
Switzerland: CCP and WHO; 2011; and Trussell J. Contraceptive failure in the United States. Contraception 2011;83:397–404.

Early Release
MMWR / June 14, 2013 / Vol. 62 7
likelihood of ovulation is low from days 1–7 of the menstrual
cycle (39). After a spontaneous or an induced abortion,
ovulation can occur within 2–3 weeks and has been found
to occur as early as 8–13 days after the end of the pregnancy.
Therefore, the likelihood of ovulation is low ≤7 days after an
abortion (40–42). A recent systematic review reported that the
mean day of first ovulation among postpartum nonlactating
women occurred 45–94 days after delivery (43). In one study,
the earliest ovulation was reported at 25 days after delivery.
Among women who are within 6 months postpartum, are fully
or nearly fully breastfeeding, and are amenorrheic, the risk for
pregnancy is <2% (44).
Although pregnancy tests often are performed before
initiating contraception, the accuracy of qualitative urine
pregnancy tests varies depending on the timing of the test
relative to missed menses, recent sexual intercourse, or recent
pregnancy. The sensitivity of a pregnancy test is defined as
the concentration of human chorionic gonadotropin (hCG)
at which 95% of tests are positive. Most qualitative pregnancy
tests approved by the U.S. Food and Drug Administration
(FDA) report a sensitivity of 20–25 mIU/mL in urine (45–48)
However, pregnancy detection rates can vary widely because of
differences in test sensitivity and the timing of testing relative
to missed menses (47,49). Some studies have shown that an
additional 11 days past the day of expected menses are needed
to detect 100% of pregnancies using qualitative tests (46). In
addition, pregnancy tests cannot detect a pregnancy resulting
from recent sexual intercourse. Qualitative tests also might have
positive results for several weeks after termination of pregnancy
because hCG can be present for several weeks after delivery or
abortion (spontaneous or induced) (50–52).
For contraceptive methods other than IUDs, the benefits
of starting to use a contraceptive method likely exceed any
risk, even in situations in which the health-care provider is
uncertain whether the woman is pregnant. Therefore, the
health-care provider can consider having patients start using
contraceptive methods other than IUDs at any time, with
a follow-up pregnancy test in 2–4 weeks. The risks of not
starting to use contraception should be weighed against the
risks of initiating contraception use in a woman who might
be already pregnant. Most studies have shown no increased
risk for adverse outcomes, including congenital anomalies
or neonatal or infant death, among infants exposed in utero
to COCs (53–55). Studies also have shown no increased risk
for neonatal or infant death or developmental abnormalities
among infants exposed in utero to DMPA (54,56,57).
In contrast, for women who want to begin using an IUD
(Cu-IUD or LNG-IUD), in situations in which the health-
care provider is uncertain whether the woman is pregnant, the
woman should be provided with another contraceptive method
to use until the health-care provider is reasonably certain that
she is not pregnant and can insert the IUD. Pregnancies among
women with IUDs are at higher risk for complications such as
spontaneous abortion, septic abortion, preterm delivery, and
chorioamnionitis (58).
A systematic review identified four analyses of data
from three diagnostic accuracy studies that evaluated the
performance of the criteria listed above through use of a
pregnancy checklist compared with a urine pregnancy test
conducted concurrently (12). The performance of the checklist
to diagnose or exclude pregnancy varied, with sensitivity
of 55%–100% and specificity of 39%–89%. The negative
predictive value was consistent across studies at 99%–100%;
the pregnancy checklist correctly ruled out women who were
not pregnant. One of the studies assessed the added usefulness
of signs and symptoms of pregnancy and found that these
criteria did not substantially improve the performance of the
pregnancy checklist, although the number of women with signs
and symptoms was small (34) (Level of evidence: Diagnostic
accuracy studies, fair, direct).
Intrauterine Contraception
Three IUDs are available in the United States, the Cu-IUD
and two LNG-IUDs (containing a total of either 13.5 mg
or 52 mg levonorgestrel). Fewer than 1 woman out of 100
becomes pregnant in the first year of using IUDs (with typical
use) (59). IUDs are long acting, are reversible, and can be
BOX 1. How To Be Reasonably Certain that a Woman Is Not Pregnant
A health-care provider can be reasonably certain that a
woman is not pregnant if she has no symptoms or signs
of pregnancy and meets any one of the following criteria:
• is ≤7 days after the start of normal menses
• has not had sexual intercourse since the start of last
normal menses
• has been correctly and consistently using a reliable
method of contraception
• is ≤7 days after spontaneous or induced abortion
•
is within 4 weeks postpartum
• is fully or nearly fully breastfeeding (exclusively
breastfeeding or the vast majority [≥85%] of feeds are
breastfeeds),* amenorrheic, and <6 months
postpartum
* Source: Labbok M, Perez A, Valdez V, et al. The Lactational Amenorrhea
Method (LAM): a postpartum introductory family planning method with
policy and program implications. Adv Contracept 1994;10:93–109.

Early Release
8 MMWR / June 14, 2013 / Vol. 62
used by women of all ages, including adolescents, and both by
parous and nulliparous women. IUDs do not protect against
STDs; consistent and correct use of male latex condoms reduces
the risk for STDs, including HIV.
Initiation of Cu-IUDs
Timing
• TheCu-IUDcanbeinsertedatanytimeifitisreasonably
certain that the woman is not pregnant (Box 1).
• TheCu-IUDalsocanbeinsertedwithin5daysofthefirst
act of unprotected sexual intercourse as an emergency
contraceptive. If the day of ovulation can be estimated, the
Cu-IUD also can be inserted >5 days after sexual intercourse
as long as insertion does not occur >5 days after ovulation.
Need for Back-Up Contraception
• Noadditionalcontraceptive protectionisneededafter
Cu-IUD insertion.
Special Considerations
Amenorrhea (Not Postpartum)
• Timing: The Cu-IUD can be inserted at any time if it is
reasonably certain that the woman is not pregnant (Box 1).
• Need for back-up contraception: No additional contraceptive
protection is needed.
Postpartum (Including After Cesarean Section)
• Timing: The Cu-IUD can be inserted at any time postpartum,
including immediately postpartum (U.S. MEC 1 or 2) (Box 2),
if it is reasonably certain that the woman is not pregnant
(Box 1). The Cu-IUD should not be inserted in a woman with
puerperal sepsis (U.S. MEC 4).
• Need for back-up contraception: No additional
contraceptive protection is needed.
Postabortion (Spontaneous or Induced)
• Timing: The Cu-IUD can be inserted within the first
7 days, including immediately postabortion (U.S. MEC 1
for first trimester abortion and U.S. MEC 2 for second
trimester abortion). The Cu-IUD should not be inserted
immediately after septic abortion (U.S. MEC 4).
• Need for back-up contraception: No additional
contraceptive protection is needed.
Switching from Another Contraceptive Method
• Timing: The Cu-IUD can be inserted immediately if it is
reasonably certain that the woman is not pregnant (Box 1).
Waiting for her next menstrual period is unnecessary.
• Need for back-up contraception: No additional
contraceptive protection is needed.
Comments and Evidence Summary. In situations in which
the health-care provider is not reasonably certain that the
woman is not pregnant, the woman should be provided with
another contraceptive method to use until the health-care
provider can be reasonably certain that she is not pregnant
and can insert the Cu-IUD.
A systematic review identified eight studies that suggested that
timing of Cu-IUD insertion in relation to the menstrual cycle in
nonpostpartum women had little effect on long-term outcomes
(rates of continuation, removal, expulsion, or pregnancy) or on
short-term outcomes (pain at insertion, bleeding at insertion, or
immediate expulsion) (13) (Level of evidence: II-2, fair, direct).
Initiation of LNG-IUDs
Timing of LNG-IUD Insertion
• TheLNG-IUDcanbeinsertedatanytimeifitisreasonably
certain that the woman is not pregnant (Box 1).
Need for Back-Up Contraception
• IftheLNG-IUDisinsertedwithinthefirst7dayssince
menstrual bleeding started, no additional contraceptive
protection is needed.
• IftheLNG-IUDisinserted>7dayssincemenstrualbleeding
started, the woman needs to abstain from sexual intercourse
or use additional contraceptive protection for the next 7 days.
BOX 2. Categories of medical eligibility criteria for contraceptive use
U.S. MEC 1 = A condition for which there is no restriction
for the use of the contraceptive method.
U.S. MEC 2 = A condition for which the advantages of
using the method generally outweigh the theoretical or
proven risks.
U.S. MEC 3 = A condition for which the theoretical or
proven risks usually outweigh the advantages of using the
method.
U.S. MEC 4 = A condition that represents an unacceptable
health risk if the contraceptive method is used.
Abbreviations: U.S. MEC = U.S. Medical Eligibility Criteria for Contraceptive
Use, 2010.
Source: CDC. U.S. medical eligibility criteria for contraceptive use.
MMWR 2010;59(No. RR-4).
Early Release
MMWR / June 14, 2013 / Vol. 62 9
Special Considerations
Amenorrhea (Not Postpartum)
• Timing: The LNG-IUD can be inserted at any time if it is
reasonably certain that the woman is not pregnant (Box 1).
• Need for back-up contraception: The woman needs to
abstain from sexual intercourse or use additional
contraceptive protection for the next 7 days.
Postpartum (Including After Cesarean Section)
•
Timing: The LNG-IUD can be inserted at any time,
including immediately postpartum (U.S. MEC 1 or 2) if
it is reasonably certain that the woman is not pregnant
(Box 1). The LNG-IUD should not be inserted in a
woman with puerperal sepsis (U.S. MEC 4).
• Need for back-up contraception: If the woman is
<6 months postpartum, amenorrheic, and fully or nearly
fully breastfeeding (exclusively breastfeeding or the vast
majority [≥85%] of feeds are breastfeeds) (60), no
additional contraceptive protection is needed. Otherwise,
a woman who is ≥21 days postpartum and has not
experienced return of her menstrual cycle needs to abstain
from sexual intercourse or use additional contraceptive
protection for the next 7 days. If her menstrual cycles have
returned and it has been >7 days since menstrual bleeding
began, she needs to abstain from sexual intercourse or use
additional contraceptive protection for the next 7 days.
Postabortion (Spontaneous or Induced)
• Timing: The LNG-IUD can be inserted within the first
7 days, including immediately postabortion (U.S. MEC 1
for first-trimester abortion and U.S. MEC 2 for second-
trimester abortion). The LNG-IUD should not be inserted
immediately after a septic abortion (U.S. MEC 4).
• Need for back-up contraception: The woman needs to
abstain from sexual intercourse or use additional
contraceptive protection for the next 7 days unless the
IUD is placed at the time of a surgical abortion.
Switching from Another Contraceptive Method
• Timing: The LNG-IUD can be inserted immediately if it
is reasonably certain that the woman is not pregnant (Box 1).
Waiting for her next menstrual period is unnecessary.
• Need for back-up contraception: If it has been >7 days
since menstrual bleeding began, the woman needs to
abstain from sexual intercourse or use additional
contraceptive protection for the next 7 days.
• Switching from a Cu-IUD: If the woman has had sexual
intercourse since the start of her current menstrual cycle
and it has been >5 days since menstrual bleeding started,
theoretically, residual sperm might be in the genital tract,
which could lead to fertilization if ovulation occurs. A
health-care provider can consider providing ECPs at the
time of LNG-IUD insertion.
Comments and Evidence Summary. In situations in which
the health-care provider is uncertain whether the woman might
be pregnant, the woman should be provided with another
contraceptive method to use until the health-care provider
can be reasonably certain that she is not pregnant and can
insert the LNG-IUD. If a woman needs to use additional
contraceptive protection when switching to an LNG-IUD
from another contraceptive method, consider continuing her
previous method for 7 days after LNG-IUD insertion. No
direct evidence was found regarding the effects of inserting
LNG-IUDs on different days of the cycle on short- or long-
term outcomes (13).
Examinations and Tests Needed Before
Initiation of a Cu-IUD or an LNG-IUD
Among healthy women, few examinations or tests are needed
before initiation of an IUD (Table 2). Bimanual examination
and cervical inspection are necessary before IUD insertion. A
baseline weight and BMI measurement might be useful for
monitoring IUD users over time. If a woman has not been
screened for STDs according to STD screening guidelines,
screening can be performed at the time of insertion. Women
with known medical problems or other special conditions
might need additional examinations or tests before being
determined to be appropriate candidates for a particular
method of contraception. U.S. MEC might be useful in such
circumstances (5).
Comments and Evidence Summary. Weight (BMI):
Obese women can use IUDs (U.S. MEC 1) (5); therefore,
screening for obesity is not necessary for the safe initiation
of IUDs. However, measuring weight and calculating BMI
(weight [kg] / height [m]
2
) at baseline might be helpful for
monitoring any changes and counseling women who might
be concerned about weight change perceived to be associated
with their contraceptive method.
Bimanual examination and cervical inspection: Bimanual
examination and cervical inspection are necessary before IUD
insertion to assess uterine size and position and to detect any
cervical or uterine abnormalities that might indicate infection
or otherwise prevent IUD insertion (61,62).
STDs: Women should be routinely screened for chlamydial
infection and gonorrhea according to national screening
guidelines. The CDC Sexually Transmitted Diseases Treatment
Guidelines provide information on screening eligibility, timing,
and frequency of screening and on screening for persons
Early Release
10 MMWR / June 14, 2013 / Vol. 62
with risk factors (32). If STD screening guidelines have been
followed, most women do not need additional STD screening
at the time of IUD insertion. If a woman has not been screened
according to guidelines, screening can be performed at the time
of IUD insertion and insertion should not be delayed. Women
with purulent cervicitis or current chlamydial infection or
gonorrhea should not undergo IUD insertion (U.S. MEC 4).
Women who have a very high individual likelihood of STD
exposure (e.g., those with a currently infected partner) generally
should not undergo IUD insertion (U.S. MEC 3) (5). For these
women, IUD insertion should be delayed until appropriate
testing and treatment occur. A systematic review did not
identify any evidence regarding women who were screened
versus not screened for STDs before IUD insertion (14).
Although women with STDs at the time of IUD insertion
have a higher risk for PID, the overall rate of PID among all
IUD users is low (63,64).
Hemoglobin: Women with iron-deficiency anemia can use
the LNG-IUD (U.S. MEC 1) (5); therefore, screening for
anemia is not necessary for safe initiation of the LNG-IUD.
Women with iron-deficiency anemia generally can use the
Cu-IUD (U.S. MEC 2). Measurement of hemoglobin before
initiation of Cu-IUDs is not necessary because of the minimal
change in hemoglobin among women with and without anemia
using Cu-IUDs. A systematic review identified four studies that
provided direct evidence for changes in hemoglobin among
women with anemia who received Cu-IUDs (30). Evidence
from one randomized trial (65) and one prospective cohort
study (66) showed no significant changes in hemoglobin
among Cu-IUD users with anemia, whereas two prospective
cohort studies (67,68) showed a statistically significant decrease
in hemoglobin levels during 12 months of follow-up; however,
the magnitude of the decrease was small and most likely not
clinically significant. The systematic review also identified 21
studies that provided indirect evidence by examining changes
in hemoglobin among healthy women receiving Cu-IUDs
(69–89), which generally showed no clinically significant
changes in hemoglobin levels with up to 5 years of follow-up
(Level of evidence: I to II-2, fair, direct).
Liver enzymes: Women with liver disease can use the
Cu-IUD (U.S. MEC 1) (5); therefore, screening for liver
disease is not necessary for the safe initiation of the Cu-IUD.
Although women with certain liver diseases generally should
not use the LNG-IUD (U.S. MEC 3) (5), screening for liver
disease before initiation of the LNG-IUD is not necessary
because of the low prevalence of these conditions and the
high likelihood that women with liver disease already would
have had the condition diagnosed. A systematic review did
not identify any evidence regarding outcomes among women
who were screened versus not screened with liver enzyme tests
before initiation of hormonal contraceptive use (14). The
prevalence of liver disorders among women of reproductive
age is low. In 2008, among adults aged 18–44 years, the
percentage with liver disease (not further specified) was 1.0%
(90). In 2009, the incidence of acute hepatitis A, B, or C
among women was <1 per 100,000 population (91). During
1998–2007, the incidence of liver carcinoma among women
was approximately 3 per 100,000 population (92). Because
estrogen and progestins are metabolized in the liver, the use
of hormonal contraceptives among women with liver disease
TABLE 2. Classification of examinations and tests needed before IUD
insertion
Examination or test
Class*
Copper-
containing IUD
Levonorgestrel-
releasing IUD
Examinations
Blood pressure C C
Weight (BMI) (weight [kg]/
height [m]
2
)
—
†
—
†
Clinical breast examination C C
Bimanual examination and
cervical inspection
A A
Laboratory tests
Glucose C C
Lipids C C
Liver enzymes C C
Hemoglobin C C
Thrombogenic mutations C C
Cervical cytology
(Papanicolaou smear)
C C
STD screening with
laboratory tests
—
§
—
§
HIV screening with laboratory
tests
C C
Abbreviations: BMI=body mass index; HIV=human immunodeficiency virus;
IUD=intrauterine device; STD=sexually transmitted disease; U.S. MEC=U.S.
Medical Eligibility Criteria for Contraceptive Use, 2010.
*
Class A: essential and mandatory in all circumstances for safe and effective
use of the contraceptive method. Class B: contributes substantially to safe
and effective use, but implementation may be considered within the public
health and/or service context; the risk of not performing an examination or
test should be balanced against the benefits of making the contraceptive
method available. Class C: does not contribute substantially to safe and
effective use of the contraceptive method.
†
Weight (BMI) measurement is not needed to determine medical eligibility for any
methods of contraception because all methods can be used (U.S. MEC 1) or
generally can be used (U.S. MEC 2) among obese women (Box 2). However,
measuring weight and calculating BMI at baseline might be helpful for monitoring
any changes and counseling women who might be concerned about weight
change perceived to be associated with their contraceptive method.
§
Most women do not require additional STD screening at the time of IUD
insertion if they have already been screened according to CDC’s STD Treatment
Guidelines (available at http://www.cdc.gov/std/treatment). If a woman has
not been screened according to guidelines, screening can be performed at
the time of IUD insertion, and insertion should not be delayed. Women with
purulent cervicitis or current chlamydial infection or gonorrhea should not
undergo IUD insertion (U.S. MEC 4). Women who have a very high individual
likelihood of STD exposure (e.g., those with a currently infected partner)
generally should not undergo IUD insertion (U.S. MEC 3). For these women,
IUD insertion should be delayed until appropriate testing and treatment occur.
Early Release
MMWR / June 14, 2013 / Vol. 62 11
might, theoretically, be a concern. The use of hormonal
contraceptives, specifically COCs and POPs, does not affect
disease progression or severity in women with hepatitis,
cirrhosis, or benign focal nodular hyperplasia (93,94), although
evidence is limited, and no evidence exists for the LNG-IUD.
Clinical breast examination: Women with breast disease
can use the Cu-IUD (U.S. MEC 1) (5); therefore, screening
for breast disease is not necessary for the safe initiation of
the Cu-IUD. Although women with current breast cancer
should not use the LNG-IUD (U.S. MEC 4) (5), screening
asymptomatic women with a clinical breast examination
before inserting an IUD is not necessary because of the low
prevalence of breast cancer among women of reproductive
age. A systematic review did not identify any evidence
regarding outcomes among women who were screened versus
not screened with a breast examination before initiation of
hormonal contraceptives (15). The incidence of breast cancer
among women of reproductive age in the United States is low.
In 2009, the incidence of breast cancer among women aged
20–49 years was approximately 72 per 100,000 women (95).
Cervical cytology: Although women with cervical cancer
should not undergo IUD insertion (U.S. MEC 4) (5),
screening asymptomatic women with cervical cytology before
IUD insertion is not necessary because of the high rates of
cervical screening, low incidence of cervical cancer in the
United States, and high likelihood that a woman with cervical
cancer already would have had the condition diagnosed. A
systematic review did not identify any evidence regarding
outcomes among women who were screened versus not
screened with cervical cytology before initiation of IUDs (14).
Cervical cancer is rare in the United States, with an incidence
rate of 8.1 per 100,000 women per year during 2004–2008
(95). The incidence and mortality rates from cervical cancer
have declined dramatically in the United States, largely because
of cervical cytology screening (96). Overall screening rates for
cervical cancer in the United States are high; among women
aged 22–30 years, approximately 87% reported having cervical
cytology screening within the last 3 years (97).
HIV screening: Although women with acquired
immunodeficiency syndrome (AIDS) who are not clinically
well should generally not undergo IUD insertion (U.S. MEC 3)
(5), HIV screening is not necessary before IUD insertion
because of the high likelihood that a woman in the United
States with such an advanced stage of disease already would
have had the condition diagnosed. A systematic review did
not identify any evidence regarding outcomes among women
who were screened versus not screened for HIV infection
before IUD insertion (14). Limited evidence suggests that
IUDs are not associated with disease progression, increased
infection, or other adverse health effects among women with
HIV infection (98).
Other screening: Women with hypertension, diabetes,
hyperlipidemia, or thrombogenic mutations can use
(U.S. MEC 1) or generally can use (U.S. MEC 2) IUDs (5).
Therefore, screening for these conditions is not necessary for
the safe initiation of IUDs.
Provision of Prophylactic Antibiotics at the
Time of IUD Insertion
• Prophylacticantibioticsaregenerallynotrecommended
for Cu-IUD or LNG-IUD insertion.
Comments and Evidence Summary. Theoretically,
IUD insertion could induce bacterial spread and lead to
complications such as PID or infective endocarditis. A
metaanalysis was conducted of randomized controlled
trials examining antibiotic prophylaxis versus placebo or
no treatment for IUD insertion (99). Use of prophylaxis
reduced the frequency of unscheduled return visits but did not
significantly reduce the incidence of PID or premature IUD
discontinuation. Although the risk for PID was higher within
the first 20 days after insertion, the incidence of PID was low
among all women who had IUDs inserted (63). In addition,
the American Heart Association recommends that the use of
prophylactic antibiotics solely to prevent infective endocarditis
is not needed for genitourinary procedures (100). Studies have
not demonstrated a conclusive link between genitourinary
procedures and infective endocarditis or a preventive benefit
of prophylactic antibiotics during such procedures (100).
Routine Follow-Up After IUD Insertion
These recommendations address when routine follow-up is
needed for safe and effective continued use of contraception
for healthy women. The recommendations refer to general
situations and might vary for different users and different
situations. Specific populations that might benefit from more
frequent follow-up visits include adolescents, persons with
certain medical conditions or characteristics, and persons with
multiple medical conditions.
• Adviseawomantoreturnatanytimetodiscusssideeffectsor
other problems, if she wants to change the method being used,
and when it is time to remove or replace the contraceptive
method. No routine follow-up visit is required.
• Atotherroutinevisits,health-careproviderswhoseeIUD
users should do the following:
– Assess the woman’s satisfaction with her contraceptive
method and whether she has any concerns about
method use.
Early Release
12 MMWR / June 14, 2013 / Vol. 62
– Assess any changes in health status, including
medications, that would change the appropriateness of
the IUD for safe and effective continued use on the
basis of U.S. MEC (e.g., category 3 and 4 conditions
and characteristics).
– Consider performing an examination to check for the
presence of the IUD strings.
– Consider assessing weight changes and counseling women
who are concerned about weight changes perceived to be
associated with their contraceptive method.
Comments and Evidence Summary. Evidence from a
systematic review about the effect of a specific follow-up visit
schedule on IUD continuation is very limited and of poor
quality. The evidence did not suggest that greater frequency of
visits or earlier timing of the first follow-up visit after insertion
improves continuation of use (16) (Level of evidence: II-2,
poor, direct). Evidence from four studies from a systematic
review on the incidence of PID among IUD initiators, or
IUD removal as a result of PID, suggested that the incidence
of PID did not differ between women using Cu-IUDs and
those using DMPA, COCs, or LNG-IUDs (17) (Level of
evidence: I to II-2, good, indirect). Evidence on the timing of
PID after IUD insertion is mixed. Although the rate of PID
was generally low, the largest study suggested that the rate of
PID was significantly higher in the first 20 days after insertion
(63) (Level of evidence: I to II-3, good to poor, indirect).
Bleeding Irregularities with Cu-IUD Use
• BeforeCu-IUDinsertion, providecounselingabout
potential changes in bleeding patterns during Cu-IUD
use. Unscheduled spotting or light bleeding, as well as
heavy or prolonged bleeding, is common during the first
3–6 months of Cu-IUD use, is generally not harmful,
and decreases with continued Cu-IUD use.
• Ifclinicallyindicated,consideranunderlyinggynecological
problem, such as Cu-IUD displacement, an STD,
pregnancy, or new pathologic uterine conditions (e.g.,
polyps or fibroids), especially in women who have already
been using the Cu-IUD for a few months or longer and
who have developed a new onset of heavy or prolonged
bleeding. If an underlying gynecological problem is found,
treat the condition or refer for care.
• Ifanunderlyinggynecologicalproblemisnotfoundand
the woman requests treatment, the following treatment
option can be considered during days of bleeding:
– Nonsteroidal antiinflammatory drugs (NSAIDs) for
short-term treatment (5–7 days)
• Ifbleedingpersistsandthewomanfindsitunacceptable,
counsel her on alternative contraceptive methods, and
offer another method if it is desired.
Comments and Evidence Summary. During contraceptive
counseling and before insertion of the Cu-IUD, information
about common side effects such as unscheduled spotting or
light bleeding or heavy or prolonged menstrual bleeding,
especially during the first 3–6 months of use, should be
discussed (70). These bleeding irregularities are generally
not harmful. Enhanced counseling about expected bleeding
patterns and reassurance that bleeding irregularities are
generally not harmful has been shown to reduce method
discontinuation in clinical trials with other contraceptives (i.e.,
DMPA) (101,102).
Evidence is limited on specific drugs, doses, and durations
of use for effective treatments for bleeding irregularities with
Cu-IUD use; therefore, although this document includes
general recommendations for treatments to consider, evidence
for specific regimens is lacking.
A systematic review identified 11 articles that examined
various therapeutic treatments for heavy menstrual bleeding,
prolonged menstrual bleeding, or both among women using
Cu-IUDs (18). Nine studies examined the use of various oral
NSAIDs for the treatment of heavy or prolonged menstrual
bleeding among Cu-IUD users and compared them to either
a placebo or a baseline cycle. Three of these trials examined
the use of indomethacin (103–105), another three examined
mefenamic acid (106–108), and another three examined
flufenamic acid (103,104,109). Other NSAIDs used in the
reported trials included alclofenac (103,104), suprofen (110),
and diclofenac sodium (111). All but one NSAID study (107)
demonstrated statistically significant or notable reductions in
mean total menstrual blood loss with NSAID use. One study
among 19 Cu-IUD users with heavy bleeding suggested that
treatment with oral tranexamic acid can significantly reduce
mean blood loss during treatment compared with placebo
(111). Data regarding the overall safety of tranexamic acid
are limited; an FDA warning states that tranexamic acid
is contraindicated in women with active thromboembolic
disease or with a history or intrinsic risk for thrombosis
or thromboembolism (112,113). Treatment with aspirin
demonstrated no statistically significant change in mean blood
loss among women whose pretreatment menstrual blood loss
was >80 mL or 60–80 mL; treatment resulted in a significant
increase among women whose pretreatment menstrual
blood loss was <60 mL (114). One study examined the use
of a synthetic form of vasopressin, intranasal desmopressin
(300
µg/day), for the first 5 days of menses for three treatment
cycles and found a significant reduction in mean blood loss
Early Release
MMWR / June 14, 2013 / Vol. 62 13
compared with baseline (106) (Level of evidence: I to II-3,
poor to fair, direct). Only one small study examined treatment
of spotting with three separate NSAIDs and did not observe
improvements in spotting in any of the groups (103) (Level
of evidence: I, poor, direct).
Bleeding Irregularities (Including
Amenorrhea) with LNG-IUD Use
• Before LNG-IUD insertion, providecounselingabout
potential changes in bleeding patterns during LNG-IUD
use. Unscheduled spotting or light bleeding is expected
during the first 3–6 months of LNG-IUD use, is generally
not harmful, and decreases with continued LNG-IUD
use. Over time, bleeding generally decreases with LNG-
IUD use, and many women experience only light
menstrual bleeding or amenorrhea. Heavy or prolonged
bleeding, either unscheduled or menstrual, is uncommon
during LNG-IUD use.
Irregular Bleeding (Spotting, Light Bleeding, or
Heavy or Prolonged Bleeding)
• Ifclinicallyindicated,consideranunderlyinggynecological
problem, such as LNG-IUD displacement, an STD,
pregnancy, or new pathologic uterine conditions (e.g.,
polyps or fibroids). If an underlying gynecological problem
is found, treat the condition or refer for care.
• Ifbleedingpersistsandthewomanfindsitunacceptable,
counsel her on alternative contraceptive methods, and
offer another method if it is desired.
Amenorrhea
• Amenorrheadoesnotrequire any medical treatment.
Provide reassurance.
– If a woman’s regular bleeding pattern changes abruptly
to amenorrhea, consider ruling out pregnancy if
clinically indicated.
• Ifamenorrheapersistsandthewomanfindsitunacceptable,
counsel her on alternative contraceptive methods, and
offer another method if it is desired
Comments and Evidence Summary. During contraceptive
counseling and before insertion of the LNG-IUD, information
about common side effects such as unscheduled spotting
or light bleeding, especially during the first 3–6 months of
use, should be discussed. Approximately half of LNG-IUD
users are likely to experience amenorrhea or oligomenorrhea
by 2 years of use (115). These bleeding irregularities are
generally not harmful. Enhanced counseling about expected
bleeding patterns and reassurance that bleeding irregularities
are generally not harmful has been shown to reduce method
discontinuation in clinical trials with other hormonal
contraceptives (i.e., DMPA) (101,102). No direct evidence
was found regarding therapeutic treatments for bleeding
irregularities during LNG-IUD use.
Management of the IUD when a Cu-IUD or
an LNG-IUD User Is Found To Have PID
• TreatthePIDaccordingtotheCDCSexually Transmitted
Diseases Treatment Guidelines (32).
• ProvidecomprehensivemanagementforSTDs,including
counseling about condom use.
• TheIUDdoesnotneedtoberemovedimmediatelyifthe
woman needs ongoing contraception.
• Reassess the woman in 48–72hours.Ifnoclinical
improvement occurs, continue antibiotics and consider
removal of the IUD.
• If the woman wantstodiscontinue use, removetheIUD
sometime after antibiotics have been started to avoid the potential
risk for bacterial spread resulting from the removal procedure.
• If the IUD is removed,considerECPs if appropriate.
Counsel the woman on alternative contraceptive methods,
and offer another method if it is desired.
• AsummaryofIUDmanagementinwomenwithPIDis
provided (Appendix F).
Comments and Evidence Summary. Treatment outcomes
do not generally differ between women with PID who retain
the IUD and those who have the IUD removed; however,
appropriate antibiotic treatment and close clinical follow-up
are necessary.
A systematic review identified four studies that included
women using copper or nonhormonal IUDs who developed
PID and compared outcomes between women who had the
IUD removed or did not (19). One randomized trial showed
that women with IUDs removed had longer hospitalizations
than those who did not, although no differences in PID
recurrences or subsequent pregnancies were observed (116).
Another randomized trial showed no differences in laboratory
findings among women who removed the IUD compared
with those who did not (117). One prospective cohort study
showed no differences in clinical or laboratory findings during
hospitalization; however, the IUD removal group had longer
hospitalizations (118). One randomized trial showed that
the rate of recovery for most clinical signs and symptoms
was higher among women who had the IUD removed than
among women who did not (119). No evidence was found
regarding women using LNG-IUDs (Level of evidence: I to
II-2, fair, direct).
Early Release
14 MMWR / June 14, 2013 / Vol. 62
Management of the IUD when a Cu-IUD or
an LNG-IUD User Is Found To Be Pregnant
• Evaluateforpossibleectopicpregnancy.
• Advisethewomanthatshehas an increased risk for
spontaneous abortion (including septic abortion that
might be life threatening) and of preterm delivery if the
IUD is left in place. The removal of the IUD reduces these
risks but might not decrease the risk to the baseline level
of a pregnancy without an IUD.
– If she does not want to continue the pregnancy, counsel
her about options.
– If she wants continue the pregnancy, advise her to seek
care promptly if she has heavy bleeding, cramping, pain,
abnormal vaginal discharge, or fever.
IUD Strings Are Visible or Can Be Retrieved Safely
from the Cervical Canal
• AdvisethewomanthattheIUDshouldberemovedas
soon as possible.
– If the IUD is to be removed, remove it by pulling on
the strings gently.
– Advise the woman that she should return promptly if
she has heavy bleeding, cramping, pain, abnormal
vaginal discharge, or fever.
• IfshechoosestokeeptheIUD,advisehertoseekcare
promptly if she has heavy bleeding, cramping, pain,
abnormal vaginal discharge, or fever.
IUD Strings Are Not Visible and Cannot Be
Retrieved Safely
• If ultrasonography is available, consider performing or
referring for ultrasound examination to determine the
location of the IUD. If the IUD cannot be located, it might
have been expelled or have perforated the uterine wall.
• IfultrasonographyisnotpossibleortheIUDisdetermined
by ultrasound to be inside the uterus, advise the woman
to seek care promptly if she has heavy bleeding, cramping,
pain, abnormal vaginal discharge, or fever.
Comments and Evidence Summary. Removing the IUD
improves the pregnancy outcome if the IUD strings are visible
or the device can be retrieved safely from the cervical canal.
Risks for spontaneous abortion, preterm delivery, and infection
are substantial if the IUD is left in place.
Theoretically, the fetus might be affected by hormonal
exposure from an LNG-IUD; however, whether this exposure
increases the risk for fetal abnormalities is unknown.
A systematic review identified nine studies suggesting that
women who did not remove their IUDs during pregnancy
were at greater risk for adverse pregnancy outcomes (including
spontaneous abortion, septic abortion, preterm delivery, and
chorioamnionitis) compared with women who had their IUDs
removed or who did not have an IUD (58). Cu-IUD removal
decreased risks but not to the baseline risk for pregnancies
without an IUD. One case series examined LNG-IUDs.
When they were not removed, eight in 10 pregnancies ended
in spontaneous abortions (Level of evidence: II-2, fair, direct).
Implants
The etonogestrel implant, a single rod with 68 mg of
etonogestrel, is available in the United States. Fewer than 1
woman out of 100 become pregnant in the first year of use of
the etonogestrel implant with typical use (59). The implant is
long acting, is reversible, and can be used by women of all ages,
including adolescents. The implant does not protect against
STDs; consistent and correct use of male latex condoms reduces
the risk for STDs, including HIV.
Initiation of Implants
Timing
• Theimplantcanbeinsertedatanytimeifitisreasonably
certain that the woman is not pregnant (Box 1).
Need for Back-Up Contraception
• If the implant isinserted within the first 5dayssince
menstrual bleeding started, no additional contraceptive
protection is needed.
• Iftheimplantisinserted>5dayssincemenstrualbleeding
started, the woman needs to abstain from sexual intercourse
or use additional contraceptive protection for the next 7 days.
Special Considerations
Amenorrhea (Not Postpartum)
• Timing: The implant can be inserted at any time if it is
reasonably certain that the woman is not pregnant (Box 1).
• Need for back-up contraception: The woman needs to
abstain from sexual intercourse or use additional
contraceptive protection for the next 7 days.
Postpartum (Breastfeeding)
• Timing: The implant can be inserted at any time (U.S.
MEC 2 if <1 month postpartum and U.S. MEC 1 if ≥1
month postpartum) if it is reasonably certain that the
woman is not pregnant (Box 1).
• Need for back-up contraception: If the woman is <6 months
postpartum, amenorrheic, and fully or nearly fully breastfeeding
(exclusively breastfeeding or the vast majority [≥85%] of feeds

Early Release
MMWR / June 14, 2013 / Vol. 62 15
are breastfeeds) (60), no additional contraceptive protection is
needed. Otherwise, a woman who is ≥21 days postpartum and
has not experienced return of her menstrual cycle needs to
abstain from sexual intercourse or use additional contraceptive
protection for the next 7 days. If her menstrual cycles have
returned and it has been >5 days since menstrual bleeding
started, she needs to abstain from sexual intercourse or use
additional contraceptive protection for the next 7 days.
Postpartum (Not Breastfeeding)
• Timing: The implant can be inserted at any time, including
immediately postpartum (U.S. MEC 1) if it is reasonably
certain that the woman is not pregnant (Box 1).
• Need for back-up contraception: A woman who is
≥21 days postpartum and has not experienced return of
her menstrual cycle needs to abstain from sexual
intercourse or use additional contraceptive protection for
the next 7 days. If her menstrual cycles have returned and
it has been >5 days since menstrual bleeding started, she
needs to abstain from sexual intercourse or use additional
contraceptive protection for the next 7 days.
Postabortion (Spontaneous or Induced)
• Timing: The implant can be inserted within the first 7 days,
including immediately after the abortion (U.S. MEC 1).
• Need for back-up contraception: The woman needs to
abstain from sexual intercourse or use additional
contraceptive protection for the next 7 days unless the
implant is placed at the time of a surgical abortion.
Switching from Another Contraceptive Method
• Timing: The implant can be inserted immediately if it is
reasonably certain that the woman is not pregnant (Box 1).
Waiting for her next menstrual period is unnecessary.
• Need for back-up contraception: If it has been >5 days
since menstrual bleeding started, the woman needs to
abstain from sexual intercourse or use additional
contraceptive protection for the next 7 days after insertion.
• Switching from an IUD: If the woman has had sexual
intercourse since the start of her current menstrual cycle and
it has been >5 days since menstrual bleeding started,
theoretically, residual sperm might be in the genital tract,
which could lead to fertilization if ovulation occurs. A health-
care provider may consider any of the following options:
– Advise the woman to retain the IUD for at least 7 days
after the implant is inserted and return for IUD removal.
– Advise the woman to abstain from sexual intercourse
or use barrier contraception for 7 days before removing
the IUD and switching to the new method.
– Advise the woman to use ECPs at the time of IUD removal.
Comments and Evidence Summary. In situations in which
the health-care provider is uncertain whether the woman might
be pregnant, the benefits of starting the implant likely exceed
any risk; therefore, starting the implant should be considered
at any time, with a follow-up pregnancy test in 2–4 weeks.
If a woman needs to use additional contraceptive protection
when switching to an implant from another contraceptive
method, consider continuing her previous method for 7 days
after implant insertion. No direct evidence was found regarding
the effects of starting the etonogestrel implant at different
times of the cycle.
Examinations and Tests Needed Before
Implant Insertion
Among healthy women, no examinations or tests are needed
before initiation of an implant, although a baseline weight and
BMI measurement might be useful for monitoring implant
users over time (Table 3). Women with known medical
problems or other special conditions might need additional
examinations or tests before being determined to be appropriate
candidates for a particular method of contraception. U.S. MEC
might be useful in such circumstances (5).
TABLE 3. Classification of examinations and tests needed before
implant insertion
Examination or test Class*
Examination
Blood pressure C
Weight (BMI) (weight [kg]/height [m]
2
) —
†
Clinical breast examination C
Bimanual examination and cervical inspection C
Laboratory test
Glucose C
Lipids C
Liver enzymes C
Hemoglobin C
Thrombogenic mutations C
Cervical cytology (Papanicolaou smear) C
STD screening with laboratory tests C
HIV screening with laboratory tests C
Abbreviations: BMI=body mass index; HIV=human immunodeficiency virus;
STD=sexually transmitted disease; U.S. MEC=U.S. Medical Eligibility Criteria for
Contraceptive Use, 2010.
* Class A: essential and mandatory in all circumstances for safe and effective
use of the contraceptive method. Class B: contributes substantially to safe and
effective use, but implementation may be considered within the public health
and/or service context; the risk of not performing an examination or test should
be balanced against the benefits of making the contraceptive method
available. Class C: does not contribute substantially to safe and effective use
of the contraceptive method.
†
Weight (BMI) measurement is not needed to determine medical eligibility for
any methods of contraception because all methods can be used (U.S. MEC 1) or
generally can be used (U.S. MEC 2) among obese women (Box 2). However,
measuring weight and calculating BMI at baseline might be helpful for
monitoring any changes and counseling women who might be concerned about
weight change perceived to be associated with their contraceptive method.
Early Release
16 MMWR / June 14, 2013 / Vol. 62
Comments and Evidence Summary. Weight (BMI): Obese
women can use implants (U.S. MEC 1) (5); therefore, screening
for obesity is not necessary for the safe initiation of implants.
However, measuring weight and calculating BMI at baseline
might be helpful for monitoring any changes and counseling
women who might be concerned about weight change perceived
to be associated with their contraceptive method.
Bimanual examination and cervical inspection: A pelvic
examination is not necessary before initiation of implants
because it would not facilitate detection of conditions for which
implant use would be unsafe. Women with current breast cancer
should not use implants (U.S. MEC 4); women with certain
liver diseases generally should not use implants (U.S. MEC 3)
(5). However, none of these conditions are likely to be detected
by pelvic examination (120). A systematic review identified
two case-control studies that compared delayed and immediate
pelvic examination before initiation of hormonal contraceptives,
specifically oral contraceptives or DMPA (15). No differences in
risk factors for cervical neoplasia, incidence of STDs, incidence
of abnormal Papanicolaou smears, or incidence of abnormal
wet mounts were observed. No evidence was found regarding
implants (Level of evidence: II-2 fair, direct).
Liver enzymes: Although women with certain liver diseases
generally should not use implants (U.S. MEC 3) (5), screening
for liver disease before initiation of implants is not necessary
because of the low prevalence of these conditions and the
high likelihood that women with liver disease already would
have had the condition diagnosed. A systematic review did
not identify any evidence regarding outcomes among women
who were screened versus not screened with liver enzyme
tests before initiation of hormonal contraceptives (14). The
prevalence of liver disorders among women of reproductive
age is low. In 2008, the percentage of adults aged 18–44
years with liver disease (not further specified) was 1.0%
(90). In 2009, the incidence of acute hepatitis A, B, or C
among women was <1 per 100,000 population (91). During
1998–2007, the incidence of liver carcinoma among women
was approximately 3 per 100,000 population (92). Because
estrogen and progestins are metabolized in the liver, the use
of hormonal contraceptives among women with liver disease
might, theoretically, be a concern. The use of hormonal
contraceptives, specifically COCs and POPs, does not affect
disease progression or severity in women with hepatitis,
cirrhosis, or benign focal nodular hyperplasia (93,94), although
evidence is limited and no evidence exists for implants.
Clinical breast examination: Although women with
current breast cancer should not use implants (U.S. MEC 4)
(5), screening asymptomatic women with a clinical breast
examination before initiating an implant is not necessary
because of the low prevalence of breast cancer among women
of reproductive age (15–49 years). A systematic review did not
identify any evidence regarding outcomes among women who
were screened versus not screened with a breast examination
before initiation of hormonal contraceptives (15). The
incidence of breast cancer among women of reproductive age
in the United States is low. In 2009, the incidence of breast
cancer among women aged 20–49 years was approximately 72
per 100,000 women (95).
Other screening: Women with hypertension, diabetes,
hyperlipidemia, anemia, thrombogenic mutations, cervical
intraepithelial neoplasia, cervical cancer, STDs, or HIV infection
can use (U.S. MEC 1) or generally can use (U.S. MEC 2)
implants (5); therefore, screening for these conditions is not
necessary for the safe initiation of implants.
Routine Follow-Up After Implant Insertion
These recommendations address when routine follow-up is
needed for safe and effective continued use of contraception
for healthy women. The recommendations refer to general
situations and might vary for different users and different
situations. Specific populations that might benefit from more
frequent follow-up visits include adolescents, those with certain
medical conditions or characteristics, and those with multiple
medical conditions.
• Adviseawomantoreturnatanytimetodiscusssideeffects
or other problems, if she wants to change the method being
used, and when it is time to remove or replace the
contraceptive method. No routine follow-up visit is required.
• Atotherroutinevisits,health-careprovidersseeingimplant
users should do the following:
– Assess the woman’s satisfaction with her contraceptive
method and whether she has any concerns about
method use.
– Assess any changes in health status, including
medications, that would change the appropriateness of
the implant for safe and effective continued use based
on U.S. MEC (e.g., category 3 and 4 conditions and
characteristics).
– Consider assessing weight changes and counseling women
who are concerned about weight changes perceived to be
associated with their contraceptive method.
Comments and Evidence Summary. A systematic review
did not identify any evidence regarding whether a routine
follow-up visit after initiating an implant improves correct or
continued use (16).
Early Release
MMWR / June 14, 2013 / Vol. 62 17
Bleeding Irregularities (Including
Amenorrhea) During Implant Use
• Before implant insertion, providecounseling about
potential changes in bleeding patterns during implant use.
Unscheduled spotting or light bleeding is common with
implant use, and some women experience amenorrhea.
These bleeding changes are generally not harmful and
might or might not decrease with continued implant use.
Heavy or prolonged bleeding, unscheduled or menstrual,
is uncommon during implant use.
Irregular Bleeding (Spotting, Light Bleeding, or
Heavy or Prolonged Bleeding)
• Ifclinicallyindicated,consideranunderlyinggynecological
problem, such as interactions with other medications, an
STD, pregnancy, or new pathologic uterine conditions
(e.g., polyps or fibroids). If an underlying gynecological
problem is found, treat the condition or refer for care.
• Ifanunderlyinggynecologicproblemisnotfoundand
the woman wants treatment, the following treatment
options during days of bleeding can be considered:
– NSAIDS for short-term treatment (5–7 days)
– Hormonal treatment (if medically eligible) with low-
dose COCs or estrogen for short-term treatment
(10–20 days)
• Ifirregularbleedingpersistsandthewoman finds it
unacceptable, counsel her on alternative methods, and
offer another method if it is desired.
Amenorrhea
• Amenorrheadoesnotrequire any medical treatment.
Provide reassurance.
– If a woman’s regular bleeding pattern changes abruptly
to amenorrhea, consider ruling out pregnancy if
clinically indicated.
• Ifamenorrheapersistsandthewomanfindsitunacceptable,
counsel her on alternative contraceptive methods, and
offer another method if it is desired.
Comments and Evidence Summary. During contraceptive
counseling and before insertion of the implant, information
about common side effects, such as unscheduled spotting or
light bleeding and amenorrhea, especially during the first
year of use should be discussed. A pooled analysis of data
from 11 clinical trials indicate that a significant proportion of
etonogestrel implant users had relatively little bleeding: 22%
of women experienced amenorrhea and 34% experienced
infrequent spotting, although 7% reported frequent bleeding
and 18% reported prolonged bleeding (121). Unscheduled
bleeding or amenorrhea is generally not harmful. Enhanced
counseling about expected bleeding patterns and reassurance
that bleeding irregularities are generally not harmful has been
shown to reduce discontinuation in clinical trials with other
hormonal contraceptives (i.e., DMPA) (101,102).
A systematic review and four newly published studies
examined several medications for the treatment of bleeding
irregularities with primarily LNG contraceptive implants
(122–126). Two small studies found significant cessation of
bleeding within 7 days of start of treatment among women
taking oral celecoxib (200 mg) daily for 5 days or oral
mefenamic acid (500 mg) 3 times daily for 5 days compared
with placebo (124,125). Differences in bleeding cessation
were not found among women with etonogestrel implants
taking mifepristone but were found when women with the
implants combined mifepristone with either ethinyl estradiol
or doxycycline (126,127). Doxycycline alone or in combination
with ethinyl estradiol did not improve bleeding cessation
among etonogestrel implant users (126). Among LNG implant
users, mifepristone reduced the number of bleeding or spotting
days but only after 6 months of treatment (128). Evidence
also suggests that estrogen (129–131), daily COCs (129),
levonorgestrel pills (130), tamoxifen (132), or tranexamic
acid (133) can reduce the number of bleeding or spotting
days during treatment among levonorgestrel implant users. In
one small study, vitamin E was found to significantly reduce
the mean number of bleeding days after the first treatment
cycle; however, another larger study reported no significant
differences in length of bleeding and spotting episodes with
vitamin E treatment (134,135). Use of aspirin did not result
in a significant difference in median length of bleeding or
bleeding and spotting episodes after treatment (134). One
study among implant users reported a reduction in number of
bleeding days after initiating ibuprofen; however, another trial
did not demonstrate any significant differences in the number
of spotting and bleeding episodes with ibuprofen compared
with placebo (123,130).
Injectables
Progestin-only injectable contraceptives (DMPA, 150 mg
intramuscularly or 104 mg subcutaneously) are available in
the United States; the only difference between these two
formulations is the route of administration. Approximately 6
out of 100 women will become pregnant in the first year of use
of DMPA with typical use (59). DMPA is reversible and can
be used by women of all ages, including adolescents. DMPA
does not protect against STDs; consistent and correct use of
male latex condoms reduces the risk for STDs, including HIV.
Early Release
18 MMWR / June 14, 2013 / Vol. 62
Initiation of Injectables
Timing
• ThefirstDMPAinjectioncanbegivenatanytimeifitis
reasonably certain that the woman is not pregnant (Box 1).
Need for Back-Up Contraception
• IfDMPAisstartedwithinthefirst7dayssincemenstrual
bleeding started, no additional contraceptive protection
is needed.
• IfDMPAisstarted>7dayssincemenstrualbleedingstarted,
the woman needs to abstain from sexual intercourse or use
additional contraceptive protection for the next 7 days.
Special Considerations
Amenorrhea (Not Postpartum)
• Timing: The first DMPA injection can be given at any
time if it is reasonably certain that the woman is not
pregnant (Box 1).
• Need for back-up contraception: The woman needs to
abstain from sexual intercourse or use additional
contraceptive protection for the next 7 days.
Postpartum (Breastfeeding)
• Timing: The first DMPA injection can be given at any
time, including immediately postpartum (U.S. MEC 2 if
<1 month postpartum and U.S. MEC 1 if ≥1 month
postpartum) if it is reasonably certain that the woman is
not pregnant (Box 1).
• Need for back-up contraception: If the woman is
<6 months postpartum, amenorrheic, and fully or nearly
fully breastfeeding (exclusively breastfeeding or the vast
majority [≥85%] of feeds are breastfeeds) (60), no
additional contraceptive protection is needed. Otherwise,
a woman who is ≥21 days postpartum and has not
experienced return of her menstrual cycle needs to abstain
from sexual intercourse or use additional contraceptive
protection for the next 7 days. If her menstrual cycles have
returned and it has been >7 days since menstrual bleeding
started, she needs to abstain from sexual intercourse or use
additional contraceptive protection for the next 7 days.
Postpartum (Not Breastfeeding)
• Timing: The first DMPA injection can be given at any
time, including immediately postpartum (U.S. MEC 1)
if it is reasonably certain that the woman is not pregnant
(Box 1).
• Need for back-up contraception: A woman who is
≥21 days postpartum and has not experienced return of
her menstrual cycle needs to abstain from sexual
intercourse or use additional contraceptive protection for
the next 7 days. If her menstrual cycles have returned and
it has been >7 days since menstrual bleeding started, she
needs to abstain from sexual intercourse or use additional
contraceptive protection for the next 7 days.
Postabortion (Spontaneous or Induced)
• Timing: The first DMPA injection can be given within
the first 7 days, including immediately postabortion
(U.S. MEC 1).
• Need for back-up contraception: The woman needs to
abstain from sexual intercourse or use additional
contraceptive protection for the next 7 days unless the
injection is given at the time of a surgical abortion.
Switching from Another Contraceptive Method
• Timing: The first DMPA injection can be given
immediately if it is reasonably certain that the woman is
not pregnant (Box 1). Waiting for her next menstrual
period is unnecessary.
• Need for back-up contraception: If it has been >7 days
since menstrual bleeding started, the woman needs to
abstain from sexual intercourse or use additional
contraceptive protection for the next 7 days.
• Switching from an IUD: If the woman has had sexual
intercourse since the start of her current menstrual cycle and
it has been >5 days since menstrual bleeding started,
theoretically, residual sperm might be in the genital tract,
which could lead to fertilization if ovulation occurs. A health-
care provider may consider any of the following options:
– Advise the women to retain the IUD for at least 7 days
after the injection and return for IUD removal.
– Advise the woman to abstain from sexual intercourse
or use barrier contraception for 7 days before removing
the IUD and switching to the new method.
– Advise the woman to use ECPs at the time of IUD removal.
Comments and Evidence Summary. In situations in which
the health-care provider is uncertain whether the woman might
be pregnant, the benefits of starting DMPA likely exceed
any risk; therefore, starting DMPA should be considered at
any time, with a follow-up pregnancy test in 2–4 weeks. If a
woman needs to use additional contraceptive protection when
switching to DMPA from another contraceptive method,
consider continuing her previous method for 7 days after
DMPA injection.
A systematic review identified eight articles examining
DMPA initiation on different days of the menstrual cycle (20).
Evidence from two studies with small samples indicated that
DMPA injections given up to day 7 of the menstrual cycle
inhibited ovulation; when DMPA was administered after

Early Release
MMWR / June 14, 2013 / Vol. 62 19
day 7, ovulation occurred in some women. Cervical mucus
was of poor quality (i.e., not favorable for sperm penetration)
in 90% of women within 24 hours of the injection (Level
of evidence: II-2, fair) (136–138). Studies found that use of
another contraceptive method until DMPA could be initiated
(bridging option) did not help women initiate DMPA and was
associated with more unintended pregnancies than immediate
receipt of DMPA (139–143) (Level of evidence: I to II-3, fair
to poor, indirect).
Examinations and Tests Needed Before
Initiation of an Injectable
Among healthy women, no examinations or tests are needed
before initiation of DMPA, although a baseline weight and
BMI measurement might be useful for monitoring DMPA users
over time (Table 4). Women with known medical problems or
other special conditions might need additional examinations
or tests before being determined to be appropriate candidates
for a particular method of contraception. U.S. MEC might
be useful in such circumstances (5).
Comments and Evidence Summary. Weight (BMI): Obese
women can use (U.S. MEC 1) or generally can use (U.S. MEC 2)
DMPA (5); therefore, screening for obesity is not necessary for
the safe initiation of DMPA. However, measuring weight and
calculating BMI at baseline might be helpful for monitoring
any changes and counseling women who might be concerned
about weight change perceived to be associated with their
contraceptive method. (See guidance on follow-up for DMPA
users for evidence on weight gain with DMPA use.)
Bimanual examination and cervical inspection: Pelvic
examination is not necessary before initiation of DMPA
because it does not facilitate detection of conditions for
which DMPA would be unsafe. Although women with
current breast cancer should not use DMPA (U.S. MEC 4),
and women with severe hypertension, heart disease, vascular
disease, migraine headaches with aura, or certain liver diseases
generally should not use DMPA (U.S. MEC 3) (5), none of
these conditions are likely to be detected by pelvic examination
(120). A systematic review identified two case-control studies
that compared delayed versus immediate pelvic examination
before initiation of hormonal contraceptives, specifically oral
contraceptives or DMPA (15). No differences in risk factors for
cervical neoplasia, incidence of STDs, incidence of abnormal
Papanicolaou smears, or incidence of abnormal wet mounts
were observed (Level of evidence: II-2, fair, direct).
Blood pressure: Women with hypertension generally can
use DMPA (U.S. MEC 2), with the exception of women with
severe hypertension or vascular disease, who generally should
not use DMPA (U.S. MEC 3) (5). Screening for hypertension
before initiation of DMPA is not necessary because of the
low prevalence of undiagnosed severe hypertension and the
high likelihood that women with these conditions already
would have had them diagnosed. A systematic review did
not identify any evidence regarding outcomes among women
who were screened versus not screened with a blood pressure
measurement before initiation of progestin-only contraceptives
(21). The prevalence of undiagnosed hypertension among
women of reproductive age is low. During 1999–2008 among
women aged 20–44 years in the United States, the percentage
with diagnosed hypertension was 7.8%, and the percentage
with undiagnosed hypertension was 1.9% (144).
Glucose: Although women with complicated diabetes
generally should not use DMPA (U.S. MEC 3) (5), screening
for diabetes before initiation of DMPA is not necessary because
of the low prevalence of undiagnosed diabetes and the high
likelihood that women with complicated diabetes would
already have had the condition diagnosed. A systematic review
did not identify any evidence regarding outcomes among
women who were screened versus not screened with glucose
measurement before initiation of hormonal contraceptives
(14). The prevalence of diabetes among women of reproductive
age is low. During 1999–2008 among women aged 20–44 years
TABLE 4. Classification of examinations and tests needed before
DMPA initiation
Examination or test Class*
Examination
Blood pressure C
Weight (BMI) (weight [kg]/height [m]
2
) —
†
Clinical breast examination C
Bimanual examination and cervical inspection C
Laboratory test
Glucose C
Lipids C
Liver enzymes C
Hemoglobin C
Thrombogenic mutations C
Cervical cytology (Papanicolaou smear) C
STD screening with laboratory tests C
HIV screening with laboratory tests C
Abbreviations: BMI=body mass index; DMPA=depot medroxyprogesterone
acetate; HIV= human immunodeficiency virus; STD= sexually transmitted
disease; U.S. MEC=U.S. Medical Eligibility Criteria for Contraceptive Use, 2010.
* Class A: essential and mandatory in all circumstances for safe and effective
use of the contraceptive method. Class B: contributes substantially to safe and
effective use, but implementation may be considered within the public health
and/or service context; the risk of not performing an examination or test should
be balanced against the benefits of making the contraceptive method
available. Class C: does not contribute substantially to safe and effective use
of the contraceptive method.
†
Weight (BMI) measurement is not needed to determine medical eligibility for
any methods of contraception because all methods can be used (U.S. MEC 1) or
generally can be used (U.S. MEC 2) among obese women (Box 2). However,
measuring weight and calculating BMI at baseline might be helpful for
monitoring any changes and counseling women who might be concerned about
weight change perceived to be associated with their contraceptive method.
Early Release
20 MMWR / June 14, 2013 / Vol. 62
in the United States, the percentage with diagnosed diabetes
was 3% and the percentage with undiagnosed diabetes was
0.5% (144). Although hormonal contraceptives can have some
adverse effects on glucose metabolism in healthy and diabetic
women, the overall clinical effect is minimal (145–151).
Liver enzymes: Although women with certain liver diseases
generally should not use DMPA (U.S. MEC 3) (5), screening
for liver disease before initiation of DMPA is not necessary
because of the low prevalence of these conditions and the high
likelihood that women with liver disease already would have
had the condition diagnosed. A systematic review did not
identify any evidence regarding outcomes among women who
were screened versus not screened with liver enzyme tests before
initiation of hormonal contraceptives (14). The prevalence of
liver disorders among women of reproductive age is low. In 2008
among adults aged 18–44 years, the percentage with liver disease
(not further specified) was 1.0% (90). In 2009, the incidence of
acute hepatitis A, B, or C among women was <1 per 100,000
population (91). During 1998–2007, the incidence of liver
carcinoma among women was approximately 3 per 100,000
population (92). Because estrogen and progestins are metabolized
in the liver, the use of hormonal contraceptives among women
with liver disease might, theoretically, be a concern. The use of
hormonal contraceptives, specifically COCs and POPs, does not
affect disease progression or severity in women with hepatitis,
cirrhosis, or benign focal nodular hyperplasia (93,94), although
evidence is limited and no evidence exists for DMPA.
Clinical breast examination: Although women with current
breast cancer should not use DMPA (U.S. MEC 4) (5), screening
asymptomatic women with a clinical breast examination before
initiating DMPA is not necessary because of the low prevalence
of breast cancer among women of reproductive age. A systematic
review did not identify any evidence regarding outcomes
among women who were screened versus not screened with
a clinical breast examination before initiation of hormonal
contraceptives (15). The incidence of breast cancer among
women of reproductive age in the United States is low. In 2009,
the incidence of breast cancer among women aged 20–49 years
was approximately 72 per 100,000 women (95).
Other screening: Women with hyperlipidemia, anemia,
thrombogenic mutations, cervical intraepithelial neoplasia,
cervical cancer, HIV infection, or other STDs can use
(U.S. MEC 1) or generally can use (U.S. MEC 2) DMPA (5);
therefore, screening for these conditions is not necessary for
the safe initiation of DMPA.
Routine Follow-Up After Injectable
Initiation
These recommendations address when routine follow-up
is recommended for safe and effective continued use of
contraception for healthy women. The recommendations refer
to general situations and might vary for different users and
different situations. Specific populations that might benefit
from more frequent follow-up visits include adolescents, those
with certain medical conditions or characteristics, and those
with multiple medical conditions.
• Adviseawomantoreturnatanytimetodiscusssideeffects
or other problems, if she wants to change the method
being used, and when it is time for reinjection. No routine
follow-up visit is required.
• Atotherroutinevisits, health-careprovidersseeing
injectable users should do the following:
– Assess the woman’s satisfaction with her contraceptive method
and whether she has any concerns about method use.
– Assess any changes in health status, including
medications, that would change the appropriateness of
the injectable for safe and effective continued use based
on U.S. MEC (e.g., category 3 and 4 conditions and
characteristics).
– Consider assessing weight changes and counseling women
who are concerned about weight changes perceived to be
associated with their contraceptive method.
Comments and Evidence Summary. Although no evidence
exists regarding whether a routine follow-up visit after initiating
DMPA improves correct or continued use, monitoring weight
or BMI change over time is important for DMPA users.
A systematic review identified a limited body of evidence that
examined whether weight gain in the few months after DMPA
initiation predicted future weight gain (17). Two studies found
significant differences in weight gain or BMI at follow-up
periods ranging from 12 to 36 months between early weight
gainers (i.e., those who gained >5% of their baseline body
weight within 6 months after initiation) and those who were
not early weight gainers (152,153). The differences between
groups were more pronounced at 18, 24, and 36 months
than at 12 months. One study found that most adolescent
DMPA users who had gained >5% of their baseline weight by
3 months gained even more weight by 12 months (154) (Level
of evidence: II-2, fair, to II-3, fair, direct).
Timing of Repeat Injections
Reinjection Interval
• ProviderepeatDMPAinjectionsevery3months(13weeks).
Early Release
MMWR / June 14, 2013 / Vol. 62 21
Special Considerations
Early Injection
• TherepeatDMPAinjectioncanbegivenearlywhennecessary.
Late Injection
• TherepeatDMPAinjectioncanbegivenupto2weeks
late (15 weeks from the last injection) without requiring
additional contraceptive protection.
• Ifthewomanis>2weekslate(>15weeksfromthelastinjection)
for a repeat DMPA injection, she can have the injection if it is
reasonably certain that she is not pregnant (Box 1). She needs to
abstain from sexual intercourse or use additional contraceptive
protection for the next 7 days. She might consider the use of
emergency contraception if appropriate.
Comments and Evidence Summary. There are no time
limits on early injections; injections can be given when
necessary (e.g., when a woman cannot return at the routine
interval). WHO has extended the time that a woman can
have a late reinjection (i.e., grace period) for DMPA use from
2 weeks to 4 weeks on the basis of data from one study showing
low pregnancy rates through 4 weeks; however, the CDC
expert group did not consider the data to be generalizable to
the United States because a large proportion of women in the
study were breastfeeding. Therefore, U.S. SPR recommends
a grace period of 2 weeks.
A systematic review identified 12 studies evaluating time to
pregnancy or ovulation after the last injection of DMPA (155).
Although pregnancy rates were low during the 2-week interval
following the reinjection date and for 4 weeks following the
reinjection date, data were sparse and one study included a
large proportion of breastfeeding women (156–158). Studies
also indicated a wide variation in time to ovulation after the
last DMPA injection, with the majority ranging from 15 to
49 weeks from the last injection (159–167) (Level of evidence:
II-2, fair, direct).
Bleeding Irregularities (Including
Amenorrhea) During Injectable Use
• Before DMPA initiation,provide counseling about
potential changes in bleeding patterns during DMPA use.
Amenorrhea and unscheduled spotting or light bleeding
is common with DMPA use, and heavy or prolonged
bleeding can occur with DMPA use. These bleeding
irregularities are generally not harmful and might decrease
with continued DMPA use.
Unscheduled Spotting or Light Bleeding
• Ifclinicallyindicated,consideranunderlyinggynecological
problem, such as interactions with other medications, an
STD, pregnancy, or new pathologic uterine conditions
(e.g., polyps or fibroids). If an underlying gynecological
problem is found, treat the condition or refer for care.
• Ifanunderlyinggynecologicproblemisnotfoundand
the woman wants treatment, the following treatment
option during days of bleeding can be considered:
– NSAIDs for short-term treatment (5–7 days)
• If unscheduled spotting or light bleeding persists and the
woman finds it unacceptable, counsel her on alternative
contraceptive methods, and offer another method if it is desired.
Heavy or Prolonged Bleeding
• Ifclinicallyindicated,consideranunderlyinggynecological
problem, such as interactions with other medications, an
STD, pregnancy, or new pathologic uterine conditions
(such as fibroids or polyps). If an underlying gynecologic
problem is identified, treat the condition or refer for care.
• Ifanunderlyinggynecologicproblemisnotfoundand
the woman wants treatment, the following treatment
options during days of bleeding can be considered:
– NSAIDS for short-term treatment (5–7 days)
– Hormonal treatment (if medically eligible) with low-
dose COCs or estrogen for short-term treatment
(10–20 days)
• Ifheavyorprolongedbleedingpersistsandthewomanfinds
it unacceptable, counsel her on alternative contraceptive
methods, and offer another method if it is desired.
Amenorrhea
• Amenorrheadoesnotrequire any medical treatment.
Provide reassurance.
– If a woman’s regular bleeding pattern changes abruptly
to amenorrhea, consider ruling out pregnancy if
clinically indicated.
• Ifamenorrheapersistsandthewomanfindsitunacceptable,
counsel her on alternative contraceptive methods, and
offer another method if it is desired.
Comments and Evidence Summary. During contraceptive
counseling and before initiation of DMPA, information
about common side effects such as irregular bleeding should
be discussed. Unscheduled bleeding or spotting is common
with DMPA use (168). Additionally, amenorrhea is common
after ≥1 years of continuous use (168,169). These bleeding
irregularities are generally not harmful. Enhanced counseling
among DMPA users detailing expected bleeding patterns and
Early Release
22 MMWR / June 14, 2013 / Vol. 62
reassurance that these irregularities generally are not harmful
has been shown to reduce DMPA discontinuation in clinical
trials (101,102).
A systematic review, as well as two additional studies,
examined the treatment of bleeding irregularities during
DMPA use (122,170,171). Two small studies found significant
cessation of bleeding within 7 days of starting treatment
among women taking valdecoxib for 5 days or mefenamic
acid for 5 days compared with placebo (172,173). Treatment
with ethinyl estradiol was found to stop bleeding better
than placebo during the treatment period, although rates
of discontinuation were high, and safety outcomes were not
examined (174). In one small study among DMPA users who
had been experiencing amenorrhea for 2 months, treatment
with COCs was found to alleviate amenorrhea better than
placebo (175). No studies examined the effects of aspirin on
bleeding irregularities among DMPA users.
Combined Hormonal Contraceptives
Combined hormonal contraceptives contain both estrogen
and a progestin and include 1) COCs (various formulations),
2) a transdermal contraceptive patch (which releases 150
µg
of norelgestromin and 20
µg ethinyl estradiol daily), and
3) a vaginal contraceptive ring (which releases 120
µg
etonogestrel and 15
µg ethinyl estradiol daily). Approximately
9 out of 100 women become pregnant in the first year of use
with combined hormonal contraceptives with typical use (59).
These methods are reversible and can be used by women of all
ages. Combined hormonal contraceptives are generally used for
21–24 consecutive days, followed by 4–7 hormone-free days
(either no use or placebo pills). These methods are sometimes
used for an extended period with infrequent or no hormone-
free days. Combined hormonal contraceptives do not protect
against STDs; consistent and correct use of male latex condoms
reduces the risk for STDs, including HIV.
Initiation of Combined Hormonal
Contraceptives
Timing
• Combinedhormonalcontraceptivescanbe initiated at
any time if it is reasonably certain that the woman is not
pregnant (Box 1).
Need for Back-Up Contraception
• Ifcombinedhormonalcontraceptivesarestartedwithin
the first 5 days since menstrual bleeding started, no
additional contraceptive protection is needed.
• Ifcombinedhormonalcontraceptivesarestarted>5days
since menstrual bleeding started, the woman needs to
abstain from sexual intercourse or use additional
contraceptive protection for the next 7 days.
Special Considerations
Amenorrhea (Not Postpartum)
• Timing: Combined hormonal contraceptives can be
started at any time if it is reasonably certain that the
woman is not pregnant (Box 1).
• Need for back-up contraception: The woman needs to
abstain from sexual intercourse or use additional
contraceptive protection for the next 7 days.
Postpartum (Breastfeeding)
• Timing: Combined hormonal contraceptives can be started
when the woman is medically eligible to use the method (176)
and if it is reasonably certain that she is not pregnant. (Box 1).
– Postpartum women who are breastfeeding should not use
combined hormonal contraceptives during the first
3 weeks after delivery (U.S. MEC 4) because of concerns
about increased risk for venous thromboembolism and
generally should not use combined hormonal contraceptives
during the fourth week postpartum (U.S. MEC 3) because
of concerns about potential effects on breastfeeding
performance. Postpartum, breastfeeding women with
other risk factors for venous thromboembolism generally
should not use combined hormonal contraceptives 4–6
weeks after delivery (U.S. MEC 3).
• Need for back-up contraception: If the woman is
<6 months postpartum, amenorrheic, and fully or nearly
fully breastfeeding (exclusively breastfeeding or the vast
majority [≥85%] of feeds are breastfeeds) (60), no
additional contraceptive protection is needed. Otherwise,
a woman who is ≥21 days postpartum and has not
experienced return of her menstrual cycle needs to abstain
from sexual intercourse or use additional contraceptive
protection for the next 7 days. If her menstrual cycles have
returned and it has been >5 days since menstrual bleeding
started, she needs to abstain from sexual intercourse or use
additional contraceptive protection for the next 7 days.
Postpartum (Not Breastfeeding)
• Timing: Combined hormonal contraceptives can be started
when the woman is medically eligible (176) and if it is
reasonably certain that she is not pregnant (Box 1).
– Postpartum women should not use combined hormonal
contraceptives during the first 3 weeks after delivery
(U.S. MEC 4) because of concerns about increased risk
Early Release
MMWR / June 14, 2013 / Vol. 62 23
for venous thromboembolism. Postpartum women with
other risk factors for venous thromboembolism
generally should not use combined hormonal
contraceptives 3–6 weeks after delivery (U.S. MEC 3).
• Need for back-up contraception: A woman who is ≥21
days postpartum and whose menstrual cycles have not
returned needs to abstain from sexual intercourse or use
additional contraceptive protection for the next 7 days. If
her menstrual cycles have returned and it has been >5 days
since menstrual bleeding started, she needs to abstain from
sexual intercourse or use additional contraceptive
protection for the next 7 days.
Postabortion (Spontaneous or Induced)
• Timing: Combined hormonal contraceptives can be started
within the first 7 days after first or second trimester abortion,
including immediately postabortion (U.S. MEC 1).
• Need for back-up contraception: She needs to abstain
from sexual intercourse or use additional contraceptive
protection for the next 7 days unless combined hormonal
contraceptives are started at the time of a surgical abortion.
Switching from Another Contraceptive Method
• Timing: Combined hormonal contraceptives can be
started immediately if it is reasonably certain that the
woman is not pregnant (Box 1). Waiting for her next
menstrual period is unnecessary.
• Need for back-up contraception: If it has been >5 days
since menstrual bleeding started, she needs to abstain from
sexual intercourse or use additional contraceptive
protection for the next 7 days.
• Switching from an IUD: If the woman has had sexual
intercourse since the start of her current menstrual cycle and
it has been >5 days since menstrual bleeding started,
theoretically, residual sperm might be in the genital tract,
which could lead to fertilization if ovulation occurs. A health-
care provider may consider any of the following options:
– Advise the women to retain the IUD for at least 7 days
after combined hormonal contraceptives are initiated
and return for IUD removal.
– Advise the woman to abstain from sexual intercourse
or use barrier contraception for 7 days before removing
the IUD and switching to the new method.
– Advise the woman to use ECPs at the time of IUD removal.
Comments and Evidence Summary. In situations in which
the health-care provider is uncertain whether the woman
might be pregnant, the benefits of starting combined hormonal
contraceptives likely exceed any risk; therefore, starting
combined hormonal contraceptives should be considered at
any time, with a follow-up pregnancy test in 2–4 weeks. If a
woman needs to use additional contraceptive protection when
switching to combined hormonal contraceptives from another
contraceptive method, consider continuing her previous method
for 7 days after starting combined hormonal contraceptives.
A systematic review of 18 studies examined the effects of
starting combined hormonal contraceptives on different days
of the menstrual cycle (22). Overall, the evidence suggested
that pregnancy rates did not differ by the timing of combined
hormonal contraceptive initiation (143,177–179) (Level of
evidence: I to II-3, fair, indirect). The more follicular activity that
occurred before starting COCs, the more likely ovulation was to
occur; however, no ovulations occurred when COCs were started
at a follicle diameter of 10 mm (mean cycle day 7.6) or when the
ring was started at 13 mm (median cycle day 11) (180–189) (Level
of evidence: I to II-3, fair, indirect). Bleeding patterns and other
side effects did not vary with the timing of combined hormonal
contraceptive initiation (177,178,190–194) (Level of evidence:
I to II-2, good to poor, direct). Although continuation rates of
combined hormonal contraceptives were initially improved by
the “quick start” approach (i.e., starting on the day of the visit),
the advantage disappeared over time (178,179,190–195) (Level
of evidence: I to II-2, good to poor, direct).
Examinations and Tests Needed Before
Initiation of Combined Hormonal
Contraceptives
Among healthy women, few examinations or tests are
needed before initiation of combined hormonal contraceptives
(Table 5). Blood pressure should be measured before initiation
of combined hormonal contraceptives. Baseline weight
and BMI measurements might be useful for monitoring
combined hormonal contraceptive users over time. Women
with known medical problems or other special conditions
might need additional examinations or tests before being
determined to be appropriate candidates for a particular
method of contraception. U.S. MEC might be useful in such
circumstances (5).
Comments and Evidence Summary. Blood pressure:
Women who have more severe hypertension (systolic pressure
of ≥160 mm Hg or diastolic pressure of ≥100 mm Hg)
or vascular disease should not use combined hormonal
contraceptives (U.S. MEC 4), and women who have less
severe hypertension (systolic pressure of 140–159 mm Hg or
diastolic pressure of 90–99 mm Hg) or adequately controlled
hypertension generally should not use combined hormonal
contraceptives (U.S. MEC 3) (5). Therefore, blood pressure
should be measured before initiating combined hormonal
contraceptives. If access to health care is limited, blood pressure
measurements may be obtained in nonclinical settings, such as

Early Release
24 MMWR / June 14, 2013 / Vol. 62
pharmacies or fire stations, and reported by the woman to her
provider. Evidence suggests that cardiovascular outcomes are
worse among women who did not have their blood pressure
measured before initiating COCs.
A systematic review identified six articles from three studies
that reported cardiovascular outcomes among women who had
blood pressure measurements and women who did not have
blood pressure measurements before initiating COCs (21).
Three case-control studies showed that women who did not
have blood pressure measurements before initiating COCs
had a higher risk for acute myocardial infarction than women
who did have blood pressure measurements (196–198). Two
case-control studies showed that women who did not have
blood pressure measurements before initiating COCs had
a higher risk for ischemic stroke than women who did have
blood pressure measurements (199,200). One case-control
study showed no difference in the risk for hemorrhagic stroke
among women who initiated COCs regardless of whether their
blood pressure was measured (201). Studies that examined
hormonal contraceptive methods other than COCs were not
identified (Level of evidence: II-2, fair, direct).
Weight (BMI): Obese women generally can use combined
hormonal contraceptives (U.S. MEC 2) (5); therefore,
screening for obesity is not necessary for the safe initiation
of combined hormonal contraceptives. However, measuring
weight and calculating BMI at baseline might be helpful for
monitoring any changes and counseling women who might
be concerned about weight change perceived to be associated
with their contraceptive method.
Bimanual examination and cervical inspection: Pelvic
examination is not necessary before initiation of combined
hormonal contraceptives because it does not facilitate detection
of conditions for which hormonal contraceptives would be
unsafe. Women with certain conditions such as current breast
cancer, severe hypertension or vascular disease, heart disease,
migraine headaches with aura, and certain liver diseases, as well
as women aged ≥35 years who smoke ≥15 cigarettes per day,
should not use (U.S. MEC 4) or generally should not use (U.S.
MEC 3) combined hormonal contraceptives (5); however, none
of these conditions are likely to be detected by pelvic examination
(120). A systematic review identified two case-control studies
that compared delayed and immediate pelvic examination
before initiation of hormonal contraceptives, specifically oral
contraceptives or DMPA (15). No differences in risk factors for
cervical neoplasia, incidence of STDs, incidence of abnormal
Papanicolaou smears, or incidence of abnormal wet mounts were
found (Level of evidence: II-2 fair, direct).
Glucose: Although women with complicated diabetes
should not use (U.S. MEC 4) or generally should not use
(U.S. MEC 3) combined hormonal contraceptives, depending
on the severity of the condition (5), screening for diabetes
before initiation of hormonal contraceptives is not necessary
because of the low prevalence of undiagnosed diabetes and the
high likelihood that women with complicated diabetes already
would have had the condition diagnosed. A systematic review
did not identify any evidence regarding outcomes among
women who were screened versus not screened with glucose
measurement before initiation of hormonal contraceptives
(14). The prevalence of diabetes among women of reproductive
age is low. During 1999–2008 among women aged 20–44 years
in the United States, the percentage with diagnosed diabetes
was 3% and the percentage with undiagnosed diabetes was
0.5% (144). Although hormonal contraceptives can have some
adverse effects on glucose metabolism in healthy and diabetic
women, the overall clinical effect is minimal (145–151).
Lipids: Although some women with hyperlipidemias
generally should not use combined hormonal contraceptives
(U.S. MEC 2/3, depending on the type and severity of the
hyperlipidemia and presence of other cardiovascular risk
factors) (5), screening for hyperlipidemia before initiation of
TABLE 5. Classification of examinations and tests needed before
combined hormonal contraceptive initiation
Examination or laboratory test Class*
Examination
Blood pressure A
†
Weight (BMI) (weight [kg]/height [m]
2
) —
§
Clinical breast examination C
Bimanual examination and cervical inspection C
Laboratory test
Glucose C
Lipids C
Liver enzymes C
Hemoglobin C
Thrombogenic mutations C
Cervical cytology (Papanicolaou smear) C
STD screening with laboratory tests C
HIV screening with laboratory tests C
Abbreviations: BMI=body mass index; HIV=human immunodeficiency virus;
STD=sexually transmitted disease; U.S. MEC=U.S. Medical Eligibility Criteria for
Contraceptive Use, 2010.
* Class A: essential and mandatory in all circumstances for safe and effective
use of the contraceptive method. Class B: contributes substantially to safe and
effective use, but implementation may be considered within the public health
and/or service context; the risk of not performing an examination or test should
be balanced against the benefits of making the contraceptive method
available. Class C: does not contribute substantially to safe and effective use
of the contraceptive method.
†
In cases in which access to health care might be limited, the blood pressure
measurement can be obtained by the woman in a nonclinical setting (e.g.,
pharmacy or fire station) and self-reported to the provider.
§
Weight (BMI) measurement is not needed to determine medical eligibility for
any methods of contraception because all methods can be used (U.S. MEC 1) or
generally can be used (U.S. MEC 2) among obese women (Box 2). However,
measuring weight and calculating BMI at baseline might be helpful for
monitoring any changes and counseling women who might be concerned about
weight change perceived to be associated with their contraceptive method.
Early Release
MMWR / June 14, 2013 / Vol. 62 25
hormonal contraceptives is not necessary because of the low
prevalence of undiagnosed disease in women of reproductive
age and the low likelihood of clinically significant changes
with use of hormonal contraceptives. A systematic review
did not identify any evidence regarding outcomes among
women who were screened versus not screened with lipid
measurement before initiation of hormonal contraceptives
(14). The prevalence of hyperlipidemia among women of
reproductive age is low. During 1999–2008 among women
aged 20–44 years in the United States, approximately 10%
had hypercholesterolemia, defined as total cholesterol
≥ 240 mg/dL or currently taking lipid-lowering medications,
and the prevalence of undiagnosed hypercholesterolemia was
approximately 2% (144). Studies have shown mixed results
about the effects of hormonal methods on lipid levels, and the
clinical significance of these changes is unclear (202–204). In
addition, women with abnormal lipid levels at baseline were
not found to have increased risk for adverse changes to their
lipid profile when using hormonal methods (202).
Liver enzymes: Although women with certain liver
diseases should not use (U.S. MEC 4) or generally should
not use (U.S. MEC 3) combined hormonal contraceptives
(5), screening for liver disease before initiation of combined
hormonal contraceptives is not necessary because of the low
prevalence of these conditions and the high likelihood that
women with liver disease already would have had the condition
diagnosed. A systematic review did not identify any evidence
regarding outcomes among women who were screened versus
not screened with liver enzyme tests before initiation of
hormonal contraceptives (14). The prevalence of liver disorders
among women of reproductive age is low. In 2008 among
adults aged 18–44 years, the percentage with liver disease (not
further specified) was 1.0% (90). In 2009, the incidence of
acute hepatitis A, B, or C among women was <1 per 100,000
population (91). During 1998–2007, the incidence of liver
carcinoma among women was approximately 3 per 100,000
population (92). Because estrogen and progestins are
metabolized in the liver, the use of hormonal contraceptives
among women with liver disease might, theoretically, be a
concern. The use of hormonal contraceptives, specifically
COCs and POPs, does not affect disease progression or severity
in women with hepatitis, cirrhosis, or benign focal nodular
hyperplasia (93,94), although evidence is limited; no evidence
exists for other types of combined hormonal contraceptives.
Thrombogenic mutations: Women with thrombogenic
mutations should not use combined hormonal contraceptives
(U.S. MEC 4) (5) because of the increased risk for venous
thromboembolism (205). However, studies have shown
that universal screening for thrombogenic mutations before
initiating COCs is not cost-effective because of the rarity of
the conditions and the high cost of screening (206–208).
Clinical breast examination: Although women with
current breast cancer should not use combined hormonal
contraceptives (U.S. MEC 4) (5), screening asymptomatic
women with a clinical breast examination before initiating
combined hormonal contraceptives is not necessary because
of the low prevalence of breast cancer among women of
reproductive age. A systematic review did not identify any
evidence regarding outcomes among women who were
screened versus not screened with a breast examination before
initiation of hormonal contraceptives (15). The incidence of
breast cancer among women of reproductive age in the United
States is low. In 2009, the incidence of breast cancer among
women aged 20–49 years was approximately 72 per 100,000
women (95).
Other screening: Women with anemia, cervical intraepithelial
neoplasia, cervical cancer, HIV infection, or other STDs can
use (U.S. MEC 1) or generally can use (U.S. MEC 2) combined
hormonal contraceptives (5); therefore, screening for these
conditions is not necessary for the safe initiation of combined
hormonal contraceptives.
Number of Pill Packs that Should Be
Provided at Initial and Return Visits
• Attheinitialandreturnvisits,provideorprescribeuptoa
1-year supply of COCs (e.g., 13 28-day pill packs),
depending on the woman’s preferences and anticipated use.
• AwomanshouldbeabletoobtainCOCseasilyinthe
amount and at the time she needs them.
Comments and Evidence Summary. The more pill packs
given up to 13 cycles, the higher the continuation rates.
Restricting the number of pill packs distributed or prescribed
can result in unwanted discontinuation of the method and
increased risk for pregnancy.
A systematic review of the evidence suggested that providing
a greater number of pill packs was associated with increased
continuation (23). Studies that compared provision of one
versus 12 packs, one versus 12 or 13 packs, or three versus seven
packs found increased continuation of pill use among women
provided with more pill packs (209–211). However, one study
found that there was no difference in continuation when patients
were provided one and then three packs versus four packs all at
once (212). In addition to continuation, a greater number of
pills packs provided was associated with fewer pregnancy tests,
fewer pregnancies, and lower cost per client. However, a greater
number of pill packs (i.e., 13 packs versus three packs) also was
associated with increased pill wastage in one study (210) (Level
of evidence: I to II-2, fair, direct).
Early Release
26 MMWR / June 14, 2013 / Vol. 62
Routine Follow-Up After Combined
Hormonal Contraceptive Initiation
These recommendations address when routine follow-up
is recommended for safe and effective continued use of
contraception for healthy women. The recommendations refer
to general situations and might vary for different users and
different situations. Specific populations that might benefit
from more frequent follow-up visits include adolescents, those
with certain medical conditions or characteristics, and those
with multiple medical conditions.
• Adviseawomantoreturnatanytimetodiscusssideeffects
or other problems or if she wants to change the method
being used. No routine follow-up visit is required.
• Atotherroutinevisits,health-careprovidersseeingcombined
hormonal contraceptive users should do the following:
– Assess the woman’s satisfaction with her contraceptive
method and whether she has any concerns about
method use.
– Assess any changes in health status, including
medications, that would change the appropriateness of
combined hormonal contraceptives for safe and
effective continued use based on U.S. MEC (e.g.,
category 3 and 4 conditions and characteristics).
– Assess blood pressure.
– Consider assessing weight changes and counseling women
who are concerned about weight changes perceived to be
associated with their contraceptive method.
Comments and Evidence Summary. No evidence exists
regarding whether a routine follow-up visit after initiating combined
hormonal contraceptives improves correct or continued use.
Monitoring blood pressure is important for combined hormonal
contraceptive users. Health-care providers might consider
recommending women obtain blood pressure measurements in
nonclinical settings (e.g., pharmacy or fire station).
A systematic review identified five studies that examined the
incidence of hypertension among women who began using
a COC versus those who started a nonhormonal method
of contraception or a placebo (17). Few women developed
hypertension after initiating COCs, and studies examining
increases in blood pressure after COC initiation found mixed
results. No studies were identified that examined changes in
blood pressure among patch or vaginal ring users (Level of
evidence: I, fair, to II-2, fair, indirect).
Late or Missed Doses and Side Effects from
Combined Hormonal Contraceptive Use
For the following recommendations, a dose is considered
late when <24 hours have elapsed since the dose should have
been taken. A dose is considered missed if ≥24 hours have
elapsed since the dose should have been taken. For example,
if a COC pill was supposed to have been taken on Monday at
9:00 a.m. and is taken at 11:00 a.m., the pill is late; however,
by Tuesday morning at 11:00 a.m., Monday’s 9:00 a.m. pill
has been missed and Tuesday’s 9:00 a.m. pill is late. For COCs,
the recommendations only apply to late or missed hormonally
active pills and not to placebo pills. Recommendations are
provided for late or missed pills (Figure 2), the patch (Figure 3),
and the ring (Figure 4).
Comments and Evidence Summary. Inconsistent or
incorrect use of combined hormonal contraceptives is a major
cause of combined hormonal contraceptive failure. Extending
the hormone-free interval is considered to be a particularly risky
time to miss combined hormonal contraceptives. Seven days of
continuous combined hormonal contraceptive use is deemed
necessary to reliably prevent ovulation. The recommendations
reflect a balance between simplicity and precision of science.
Women who frequently miss COCs or experience other usage
errors with combined hormonal patch or combined vaginal
ring should consider an alternative contraceptive method
that is less dependent on the user to be effective (e.g., IUD,
implant, or injectable).
A systematic review identified 36 studies that examined
measures of contraceptive effectiveness of combined hormonal
contraceptives during cycles with extended hormone-free
intervals, shortened hormone-free intervals, or deliberate
nonadherence on days not adjacent to the hormone-free
interval (24). Most of the studies examined COCs (188,213–
240), two examined the combined hormonal patch (234,241),
and six examined the combined vaginal ring (185,242–246).
No direct evidence on the effect of missed pills on the risk
for pregnancy was found. Studies of women deliberately
extending the hormone-free interval up to 14 days found
wide variability in the amount of follicular development and
occurrence of ovulation (216,219,221,222,224,225,227–230);
in general, the risk for ovulation was low, and among women
who did ovulate, cycles were usually abnormal. In studies of
women who deliberately missed pills on various days during
the cycle not adjacent to the hormone-free interval, ovulation
occurred infrequently (214,220–222,230,231,233,234).
Studies comparing 7-day hormone-free intervals with shorter
hormone-free intervals found lower rates of pregnancy
(213,217,226,232) and significantly greater suppression of
ovulation (215,225,236–
238,240) among women with shorter
intervals in all but one study (235), which found no difference.
Two studies that compared 30-
µg ethinyl estradiol pills with
20-
µg ethinyl estradiol pills showed more follicular activity
when 20-
µg ethinyl estradiol pills were missed (216,219). In
Early Release
MMWR / June 14, 2013 / Vol. 62 27
studies examining the combined vaginal ring, three studies
found that nondeliberate extension of the hormone-free
interval for 24 to <48 hours from the scheduled period
did not increase the risk for pregnancy (242,243,245); one
study found that ring insertion after a deliberately extended
hormone-free interval that allowed a 13-mm follicle to develop
interrupted ovarian function and further follicular growth
(185); and one study found that inhibition of ovulation was
maintained after deliberately forgetting to remove the ring
for up to 2 weeks after normal ring use (246). In studies
examining the combined hormonal patch, one study found
that missing 1–3 consecutive days before patch replacement
(either wearing one patch 3 days longer before replacement
or going 3 days without a patch before replacing the next
patch) on days not adjacent to the patch-free interval resulted
in little follicular activity and low risk for ovulation (234),
and one pharmacokinetic study found that serum levels of
ethinyl estradiol and progestin norelgestromin remained within
reference ranges after extending patch wear for 3 days (241).
No studies were found on extending the patch-free interval. In
studies that provide indirect evidence on the effects of missed
combined hormonal contraception on surrogate measures of
pregnancy, how differences in surrogate measures correspond
to pregnancy risk is unclear (Level of evidence: I, good, indirect
to II-3, poor, direct).
Vomiting or Severe Diarrhea While Using COCs
Certain steps should be taken by women who experience
vomiting or severe diarrhea while using COCs (Figure 5).
Comments and Evidence Summary. Theoretically, the
contraceptive effectiveness of COCs might be decreased because
of vomiting or severe diarrhea. Because of the lack of evidence
that addresses vomiting or severe diarrhea while using COCs,
these recommendations are based on the recommendations
FIGURE 2. Recommended actions after late or missed combined oral contraceptives
If one hormonal pill is late:
(<24 hours since a pill
should have been taken)
If one hormonal pill has been
missed: (24 to <48 hours since a
pill should have been taken)
If two or more consecutive hormonal
pills have been missed: (≥48 hours since
a pill should have been taken)
• Take the late or missed pill as
soon as possible.
• Continue taking the remaining
pills at the usual time (even if it
means taking two pills on the
same day).
• No additional contraceptive
protection is needed.
• Emergency contraception is not
usually needed but can be
considered if hormonal pills
were missed earlier in the cycle
or in the last week of the
previous cycle.
• Take the most recent missed pill as
soon as possible. (Any other missed
pills should be discarded.)
• Continue taking the remaining pills at
the usual time (even if it means taking
two pills on the same day).
• Use back-up contraception (e.g.,
condoms) or avoid sexual intercourse
until hormonal pills have been taken
for 7 consecutive days.
• If pills were missed in the last week of
hormonal pills (e.g., days 15–21 for
28-day pill packs):
— Omit the hormone-free interval by
nishing the hormonal pills in the
current pack and starting a new
pack the next day.
— If unable to start a new pack
immediately, use back-up
contraception (e.g., condoms) or
avoid sexual intercourse until
hormonal pills from a new pack
have been taken for 7 consecutive
days.
• Emergency contraception should be
considered if hormonal pills were
missed during the rst week and
unprotected sexual intercourse
occurred in the previous 5 days.
• Emergency contraception may also
be considered at other times as
appropriate.
Early Release
28 MMWR / June 14, 2013 / Vol. 62
for missed COCs. No evidence was found on the effects of
vomiting or diarrhea on measures of contraceptive effectiveness
including pregnancy, follicular development, hormone levels,
or cervical mucus quality.
Unscheduled Bleeding with Extended or
Continuous Use of Combined
Hormonal Contraceptives
• Beforeinitiationofcombinedhormonalcontraceptives,
provide counseling about potential changes in bleeding
patterns during extended or continuous combined
hormonal contraceptive use. (Extended contraceptive use
is defined as a planned hormone-free interval after at least
two contiguous cycles. Continuous contraceptive use is
defined as uninterrupted use of hormonal contraception
without a hormone-free interval [247].)
• Unscheduledspottingorbleedingiscommonduringthe
first 3–6 months of extended or continuous combined
hormonal contraceptive use. It is generally not harmful
and decreases with continued combined hormonal
contraceptive use.
• Ifclinicallyindicated,consideranunderlyinggynecological
problem, such as inconsistent use, interactions with other
medications, cigarette smoking, an STD, pregnancy, or
new pathologic uterine conditions (e.g., polyps or
fibroids). If an underlying gynecological problem is found,
treat the condition or refer for care.
• Ifanunderlyinggynecologicalproblemisnotfoundand
the woman wants treatment, the following treatment
option can be considered:
– Advise the woman to discontinue combined hormonal
contraceptive use (i.e., a hormone-free interval) for 3–4
consecutive days; a hormone-free interval is not
recommended during the first 21 days of using the
continuous or extended combined hormonal
contraceptive method. A hormone-free interval also is
not recommended more than once per month because
contraceptive effectiveness might be reduced.
• Ifunscheduledspottingorbleedingpersistsandthewoman
finds it unacceptable, counsel her on alternative contraceptive
methods, and offer another method if it is desired.
Comments and Evidence Summary. During contraceptive
counseling and before initiating extended or continuous
FIGURE 3. Recommended actions after delayed application or detachment with combined hormonal patch
Delayed application or detachment* for <48
hours since a patch should have been applied
or reattached
Delayed application or detachment* for ≥48
hours since a patch should have been applied
or reattached
• Apply a new patch as soon as possible. (If
detachment occured <24 hours since the
patch was applied, try to reapply the patch
or replace with a new patch.)
• Keep the same patch change day.
• No additional contraceptive protection is
needed.
• Emergency contraception is not usually
needed but can be considered if delayed
application or detachment occurred earlier
in the cycle or in the last week of the
previous cycle.
• Apply a new patch as soon as possible.
• Keep the same patch change day.
• Use back-up contraception (e.g., condoms)
or avoid sexual intercourse until a patch has
been worn for 7 consecutive days.
• If the delayed application or detachment
occurred in the third patch week:
— Omit the hormone-free week by
nishing the third week of patch use
(keeping the same patch change day)
and starting a new patch immediately.
— If unable to start a new patch
immediately, use back-up
contraception (e.g., condoms) or avoid
sexual intercourse until a new patch has
been worn for 7 consecutive days.
• Emergency contraception should be
considered if the delayed application or
detachment occurred within the rst week
of patch use and unprotected sexual
intercourse occurred in the previous 5 days.
• Emergency contraception may also be
considered at other times as appropriate.
* If detachment takes place but the woman is unsure when the detachment occurred, consider the patch to have been detached for ≥48 hours since a patch should
have been applied or reattached.
Early Release
MMWR / June 14, 2013 / Vol. 62 29
combined hormonal contraceptives, information about
common side effects such as unscheduled spotting or bleeding,
especially during the first 3–6 months of use, should be
discussed (248). These bleeding irregularities are generally
not harmful and usually improve with persistent use of the
hormonal method. To avoid unscheduled spotting or bleeding,
counseling should emphasize the importance of correct use and
timing; for users of contraceptive pills, emphasize consistent
pill use. Enhanced counseling about expected bleeding patterns
and reassurance that bleeding irregularities are generally not
harmful has been shown to reduce method discontinuation in
clinical trials with DMPA (101,102).
A systematic review identified three studies with small study
populations that addressed treatments for unscheduled bleeding
among women using extended or continuous combined
hormonal contraceptives (25). In two separate randomized
clinical trials in which women were taking either contraceptive
pills or using the contraceptive ring continuously for 168 days,
women assigned to a hormone-free interval of 3 or 4 days
reported improved bleeding. Although they noted an initial
increase in flow, this was followed by an abrupt decrease 7–8
days later with eventual cessation of flow 11–12 days later.
These findings were compared with women who continued to
use their method without a hormone-free interval, in which a
greater proportion reported either treatment failure or fewer
days of amenorrhea (249,250). In another randomized trial of
66 women with unscheduled bleeding among women using 84
days of hormonally active contraceptive pills, oral doxycycline
(100 mg twice daily) initiated the first day of bleeding and
taken for 5 days did not result in any improvement in bleeding
compared with placebo (251) (Level of evidence: I, fair, direct).
Progestin-Only Pills
POPs contain only a progestin and no estrogen and are
available in the United States. Approximately 9 out of 100
women become pregnant in the first year of use with POPs
with typical use (59). POPs are reversible and can be used
by women of all ages. POPs do not protect against STDs;
consistent and correct use of male latex condoms reduces the
risk for STDs, including HIV.
FIGURE 4. Recommended actions after delayed insertion or reinsertion with combined vaginal ring
Delayed insertion of a new ring or delayed
reinsertion* of a current ring for <48 hours
since a ring should have been inserted
Delayed insertion of a new ring or delayed
reinsertion* for ≥48 hours since a ring should
have been inserted
• Insert ring as soon as possible.
• Keep the ring in until the scheduled ring
removal day.
• No additional contraceptive protection is
needed.
• Emergency contraception is not usually
needed but can be considered if delayed
insertion or reinsertion occurred earlier in
the cycle or in the last week of the previous
cycle.
• Insert ring as soon as possible.
• Keep the ring in until the scheduled ring
removal day.
• Use back-up contraception (e.g., condoms)
or avoid sexual intercourse until a ring has
been worn for 7 consecutive days.
• If the ring removal occurred in the third
week of ring use:
— Omit the hormone-free week by
nishing the third week of ring use and
starting a new ring immediately.
— If unable to start a new ring
immediately, use back-up contraception
(e.g., condoms) or avoid sexual
intercourse until a new ring has been
worn for 7 consecutive days.
• Emergency contraception should be
considered if the delayed insertion or
reinsertion occurred within the rst week of
ring use and unprotected sexual intercourse
occurred in the previous 5 days.
• Emergency contraception may also be
considered at other times as appropriate.
* If removal takes place but the woman is unsure of how long the ring has been removed, consider the ring to have been removed for ≥48 hours since a ring should
have been inserted or reinserted.
Early Release
30 MMWR / June 14, 2013 / Vol. 62
Initiation of POPs
Timing
• POPscanbestartedatanytimeifitisreasonablycertain
that the woman is not pregnant (Box 1).
Need for Back-Up Contraception
• IfPOPsarestartedwithinthefirst5dayssincemenstrual
bleeding started, no additional contraceptive protection
is needed.
• IfPOPsarestarted>5dayssincemenstrualbleedingstarted,
the woman needs to abstain from sexual intercourse or use
additional contraceptive protection for the next 2 days.
Special Considerations
Amenorrhea (Not Postpartum)
• Timing: POPs can be started at any time if it is reasonably
certain that the woman is not pregnant (Box 1).
• Need for back-up contraception: The woman needs to
abstain from sexual intercourse or use additional
contraceptive protection for the next 2 days.
Postpartum (Breastfeeding)
• Timing: POPs can be started at any time, including
immediately postpartum (U.S. MEC 2 if <1 month postpartum
and U.S. MEC 1 if ≥1 month postpartum) if it is reasonably
certain that the woman is not pregnant (Box 1).
• Need for back-up contraception: If the woman is
<6 months postpartum, amenorrheic, and fully or nearly
fully breastfeeding (exclusively breastfeeding or the vast
majority [≥85%] of feeds are breastfeeds) (60), no
additional contraceptive protection is needed. Otherwise,
a woman who is ≥21 days postpartum and has not
experienced return of her menstrual cycles needs to abstain
from sexual intercourse or use additional contraceptive
protection for the next 2 days. If her menstrual cycles have
FIGURE 5. Recommended steps after vomiting or diarrhea while using combined oral contraceptives
Vomiting or diarrhea (for any
reason, for any duration) that
occurs within 24 hours after
taking a hormonal pill
Vomiting or diarrhea, for any
reason, continuing for 24 to <48
hours after taking any hormonal
pill
Vomiting or diarrhea, for any reason,
continuing for ≥48 hours after taking any
hormonal pill
• Taking another hormonal pill
(redose) is unnecessary.
• Continue taking pills daily at the
usual time (if possible, despite
discomfort).
• No additional contraceptive
protection is needed.
• Emergency contraception is not
usually needed but can be
considered as appropriate.
• Continue taking pills daily at the usual
time (if possible, despite discomfort).
• Use back-up contraception (e.g.,
condoms) or avoid sexual intercourse
until hormonal pills have been taken
for 7 consecutive days after vomiting or
diarrhea has resolved.
• If vomiting or diarrhea occurred in the
last week of hormonal pills (e.g., days
15–21 for 28-day pill packs):
— Omit the hormone-free interval by
nishing the hormonal pills in the
current pack and starting a new
pack the next day.
— If unable to start a new pack
immediately, use back-up
contraception (e.g., condoms) or
avoid sexual intercourse until
hormonal pills from a new pack
have been taken for 7 consecutive
days.
• Emergency contraception should be
considered if vomiting or diarrhea
occurred within the rst week of a new
pill pack and unprotected sexual
intercourse occurred in the previous 5
days.
• Emergency contraception may also be
considered at other times as
appropriate.

Early Release
MMWR / June 14, 2013 / Vol. 62 31
returned and it has been >5 days since menstrual bleeding
started, she needs to abstain from sexual intercourse or use
additional contraceptive protection for the next 2 days.
Postpartum (Not Breastfeeding)
• Timing: POPs can be started at any time, including
immediately postpartum (U.S. MEC 1), if it is reasonably
certain that the woman is not pregnant (Box 1).
• Need for back-up contraception: Women who are
≥21 days postpartum and whose menstrual cycles have
not returned need to abstain from sexual intercourse or
use additional contraceptive protection for the next 2 days.
If her menstrual cycles have returned and it has been >5
days since menstrual bleeding started, she needs to abstain
from sexual intercourse or use additional contraceptive
protection for the next 2 days.
Postabortion (Spontaneous or Induced)
• Timing: POPs can be started within the first 7 days,
including immediately postabortion (U.S. MEC 1).
• Need for back-up contraception: The woman needs to
abstain from sexual intercourse or use additional
contraceptive protection for the next 2 days unless POPs
are started at the time of a surgical abortion.
Switching from Another Contraceptive Method
• Timing: POPs can be started immediately if it is reasonably
certain that the woman is not pregnant (Box 1). Waiting
for her next menstrual period is unnecessary.
• Need for back-up contraception: If it has been >5 days
since menstrual bleeding started, she needs to abstain from
sexual intercourse or use additional contraceptive
protection for the next 2 days.
• Switching from an IUD: If the woman has had sexual
intercourse since the start of her current menstrual cycle and
it has been >5 days since menstrual bleeding started,
theoretically, residual sperm might be in the genital tract,
which could lead to fertilization if ovulation occurs. A health-
care provider may consider any of the following options:
– Advise the women to retain the IUD for at least 2 days
after POPs are initiated and return for IUD removal.
– Advise the woman to abstain from sexual intercourse
or use barrier contraception for 2 days before removing
the IUD and switching to the new method.
– Advise the woman to use ECPs at the time of IUD removal.
Comments and Evidence Summary. In situations in which
the health-care provider is uncertain whether the woman might
be pregnant, the benefits of starting POPs likely exceed any
risk; therefore, starting POPs should be considered at any time,
with a follow-up pregnancy test in 2–4 weeks.
Unlike COCs, POPs inhibit ovulation in about half of cycles,
although the rates vary widely by individual (252). Peak serum
steroid levels are reached about 2 hours after administration,
followed by rapid distribution and elimination, such that by
24 hours after administration, serum steroid levels are near
baseline (252). Therefore, taking POPs at approximately
the same time each day is important. An estimated 48
hours of POP use has been deemed necessary to achieve the
contraceptive effects on cervical mucus (252). If a woman needs
to use additional contraceptive protection when switching to
POPs from another contraceptive method, consider continuing
her previous method for 2 days after starting POPs. No direct
evidence was found regarding the effects of starting POPs at
different times of the cycle.
Examinations and Tests Needed Before
Initiation of POPs
Among healthy women, no examinations or tests are needed
before initiation of POPs, although a baseline weight and BMI
measurement might be useful for monitoring POP users over
time (Table 6). Women with known medical problems or
other special conditions might need additional examinations
or tests before being determined to be appropriate candidates
TABLE 6. Classification of examinations and tests needed before POP
initiation
Examination or laboratory test Class*
Examination
Blood pressure C
Weight (BMI) (weight [kg]/height [m]
2
) —
†
Clinical breast examination C
Bimanual examination and cervical inspection C
Laboratory test
Glucose C
Lipids C
Liver enzymes C
Hemoglobin C
Thrombogenic mutations C
Cervical cytology (Papanicolaou smear) C
STD screening with laboratory tests C
HIV screening with laboratory tests C
Abbreviations: BMI=body mass index; HIV=human immunodeficiency virus;
POP = progestin-only pill; STD=sexually transmitted disease; U.S. MEC=U.S.
Medical Eligibility Criteria for Contraceptive Use, 2010.
*
Class A: essential and mandatory in all circumstances for safe and effective
use of the contraceptive method. Class B: contributes substantially to safe and
effective use, but implementation may be considered within the public health
and/or service context; the risk of not performing an examination or test should
be balanced against the benefits of making the contraceptive method
available. Class C: does not contribute substantially to safe and effective use
of the contraceptive method.
†
Weight (BMI) measurement is not needed to determine medical eligibility for
any methods of contraception because all methods can be used (U.S. MEC 1) or
generally can be used (U.S. MEC 2) among obese women (Box 2). However,
measuring weight and calculating BMI at baseline might be helpful for
monitoring any changes and counseling women who might be concerned about
weight change perceived to be associated with their contraceptive method.
Early Release
32 MMWR / June 14, 2013 / Vol. 62
for a particular method of contraception. U.S. MEC might
be useful in such circumstances (5).
Comments and Evidence Summary. Weight (BMI): Obese
women can use POPs (U.S. MEC 1) (5); therefore, screening
for obesity is not necessary for the safe initiation of POPs.
However, measuring weight and calculating BMI at baseline
might be helpful for monitoring any changes and counseling
women who might be concerned about weight change
perceived to be associated with their contraceptive method.
Bimanual examination and cervical inspection: Pelvic
examination is not necessary before initiation of POPs because
it does not facilitate detection of conditions for which POPs
would be unsafe. Women with current breast cancer should
not use POPs (U.S. MEC 4), and women with certain liver
diseases generally should not use POPs (U.S. MEC 3) (5);
however, neither of these conditions are likely to be detected
by pelvic examination (120). A systematic review identified
two case-control studies that compared delayed versus
immediate pelvic examination before initiation of hormonal
contraceptives, specifically oral contraceptives or DMPA (15).
No differences in risk factors for cervical neoplasia, incidence
of STDs, incidence of abnormal Papanicolaou smears, or
incidence of abnormal wet mounts were observed (Level of
evidence: II-2 fair, direct).
Liver enzymes: Although women with certain liver diseases
generally should not use POPs (U.S. MEC 3) (5), screening
for liver disease before initiation of POPs is not necessary
because of the low prevalence of these conditions and the
high likelihood that women with liver disease already would
have had the condition diagnosed. A systematic review did
not identify any evidence regarding outcomes among women
who were screened versus not screened with liver enzyme
tests before initiation of hormonal contraceptives (14). The
prevalence of liver disorders among women of reproductive
age is low. In 2008 among U.S. adults aged 18–44 years,
the percentage with liver disease (not further specified) was
1.0% (90). In 2009, the incidence of acute hepatitis A, B,
or C among women was <1 per 100,000 population (91).
During 1998–2007, the incidence of liver carcinoma among
women was approximately 3 per 100,000 population (92).
Because estrogen and progestins are metabolized in the liver,
the use of hormonal contraceptives among women with liver
disease might, theoretically, be a concern. The use of hormonal
contraceptives, specifically COCs and POPs, does not affect
disease progression or severity in women with hepatitis,
cirrhosis, or benign focal nodular hyperplasia (93,94).
Clinical breast examination: Although women with current
breast cancer should not use POPs (U.S. MEC 4) (5), screening
asymptomatic women with a clinical breast examination
before initiating POPs is not necessary because of the low
prevalence of breast cancer among women of reproductive age.
A systematic review did not identify any evidence regarding
outcomes among women who were screened versus not
screened with a clinical breast examination before initiation of
hormonal contraceptives (15). The incidence of breast cancer
among women of reproductive age in the United States is low.
In 2009, the incidence of breast cancer among women ages
20–49 was approximately 72 per 100,000 women (95).
Other screening: Women with hypertension, diabetes,
hyperlipidemia, anemia, thrombogenic mutations, cervical
intraepithelial neoplasia, cervical cancer, STDs, or HIV
infection can use (U.S. MEC 1) or generally can use (U.S.
MEC 2) POPs (5); therefore, screening for these conditions
is not necessary for the safe initiation of POPs.
Number of Pill Packs that Should Be
Provided at Initial and Return Visits
• Attheinitialandreturnvisits,provideorprescribeuptoa
1-year supply of POPs (e.g., 13 28-day pill packs),
depending on the woman’s preferences and anticipated use.
• Awoman should beable to obtain POPseasily in the
amount and at the time she needs them.
Comments and Evidence Summary. The more pill packs
given up to 13 cycles, the higher the continuation rates.
Restricting the number of pill packs distributed or prescribed
can result in unwanted discontinuation of the method and
increased risk for pregnancy.
A systematic review of the evidence suggested that providing
a greater number of pill packs was associated with increased
continuation (23). Studies that compared provision of one
versus 12 packs, one versus 12 or 13 packs, or three versus
seven packs found increased continuation of pill use among
women provided with more pill packs (209–211). However,
one study found that there was no difference in continuation
when patients were provided one and then three packs versus
four packs all at once (212). In addition to continuation, a
greater number of pill packs provided was associated with fewer
pregnancy tests, fewer pregnancies, and lower cost per client.
However, a greater number of pill packs (13 packs versus three
packs) also was associated with increased pill wastage in one
study (210) (Level of evidence: I to II-2, fair, direct).
Routine Follow-Up After POP Initiation
These recommendations address when routine follow-up
is recommended for safe and effective continued use of
contraception for healthy women. The recommendations refer
to general situations and might vary for different users and
Early Release
MMWR / June 14, 2013 / Vol. 62 33
different situations. Specific populations that might benefit
from more frequent follow-up visits include adolescents, those
with certain medical conditions or characteristics, and those
with multiple medical conditions.
• Adviseawomantoreturnatanytimetodiscusssideeffects
or other problems or if she wants to change the method
being used. No routine follow-up visit is required.
• Atotherroutinevisits,health-careprovidersseeingPOP
users should do the following:
– Assess the woman’s satisfaction with her contraceptive
method and whether she has any concerns about
method use.
– Assess any changes in health status, including medications,
that would change the appropriateness of POPs for safe
and effective continued use based on U.S. MEC (e.g.,
category 3 and 4 conditions and characteristics).
– Consider assessing weight changes and counseling women
who are concerned about weight changes perceived to be
associated with their contraceptive method.
Comments and Evidence Summary. No evidence was
found regarding whether a routine follow-up visit after
initiating POPs improves correct or continued use.
Missed POPs
For the following recommendations, a dose is considered
missed if it has been >3 hours since it should have been taken.
• Takeonepillassoonaspossible.
• Continuetakingpillsdaily,oneeachday,atthesametime
each day, even if it means taking two pills on the same day.
• Useback-upcontraception(e.g.,condoms)oravoidsexual
intercourse until pills have been taken correctly, on time,
for 2 consecutive days.
• Emergency contraception should be considered if the
woman has had unprotected sexual intercourse.
Comments and Evidence Summary. Inconsistent or
incorrect use of oral contraceptive pills is a major reason for oral
contraceptive failure. Unlike COCs, POPs inhibit ovulation
in about half of cycles, although this rate varies widely by
individual (252). Peak serum steroid levels are reached about
2 hours after administration, followed by rapid distribution
and elimination, such that by 24 hours after administration,
serum steroid levels are near baseline (252). Therefore, taking
POPs at approximately the same time each day is important.
An estimated 48 hours of POP use was deemed necessary to
achieve the contraceptive effects on cervical mucus (252).
Women who frequently miss POPs should consider an
alternative contraceptive method that is less dependent on
the user to be effective (e.g., IUD, implant, or injectable).
No evidence was found regarding the effects of missed POPs
available in the United States on measures of contraceptive
effectiveness including pregnancy, follicular development,
hormone levels, or cervical mucus quality.
Vomiting or Severe Diarrhea (for any
Reason or Duration) that Occurs Within
3 Hours After Taking a Pill
• Takeanotherpillassoonaspossible(ifpossible,despite
discomfort).
• Continuetakingpillsdaily,oneeachday,atthesametime
each day.
• Useback-up contraception (e.g., condoms) or avoid sexual
intercourse until 2 days after vomiting or diarrhea has resolved.
• Emergency contraception should be considered if the
woman has had unprotected sexual intercourse.
Comments and Evidence Summary. Theoretically, the
contraceptive effectiveness of POPs might be decreased because
of vomiting or severe diarrhea. Because of the lack of evidence
to address this question, these recommendations are based on
the recommendations for missed POPs. No evidence was found
regarding the effects of vomiting or diarrhea on measures of
contraceptive effectiveness, including pregnancy, follicular
development, hormone levels, or cervical mucus quality.
Standard Days Method
SDM is a method based on fertility awareness; users must
avoid unprotected sexual intercourse on days 8–19 of the
menstrual cycle (253). Approximately 5 out of 100 women
become pregnant in the first year of use with perfect (i.e.,
correct and consistent) use of SDM (253); effectiveness based
on typical use is not available for this method but is expected
to be lower than that for perfect use. SDM is reversible and can
be used by women of all ages. SDM does not protect against
STDs; consistent and correct use of male latex condoms reduces
the risk for STDs, including HIV.
Use of SDM Among Women with Various
Menstrual Cycle Durations
Menstrual Cycles of 26–32 Days
• Thesewomenmayusethemethod.
• Provideabarriermethodofcontraceptionforprotection
on days 8–19 if she wants one.
• Ifshehasunprotectedsexualintercourseduringdays8–19,
consider the use of emergency contraception if appropriate.
Early Release
34 MMWR / June 14, 2013 / Vol. 62
Two or More Menstrual Cycles of <26 or >32 Days
Within Any 1 Year of SDM Use
• Advisethe woman that the method might not be
appropriate for her because of a higher risk for pregnancy.
Help her consider another method.
Comments and Evidence Summary. The probability of
pregnancy is increased when the menstrual cycle is outside the
range of 26–32 days, even if unprotected sexual intercourse is
avoided on days 8–19. A study of 7,600 menstrual cycles, including
information on cycle length and signs of ovulation, concluded that
the theoretical effectiveness of SDM is greatest for women with
cycles of 26–32 days, that the method is still effective for women
who occasionally have a cycle outside this range, and that it is
less effective for women who consistently have cycles outside this
range. Information from daily hormonal measurements shows
that the timing of the 6-day fertile window varies greatly, even
among women with regular cycles (39,254,255).
Emergency Contraception
Emergency contraception consists of methods that can be
used by women after sexual intercourse to prevent pregnancy.
Emergency contraception methods have varying ranges
of effectiveness depending on the method and timing of
administration. Four options are available in the United States:
the Cu-IUD and three types of ECPs.
Types of Emergency Contraception
Intrauterine Device
• Cu-IUD
ECPs
• Ulipristalacetate(UPA)inasingledose(30mg)
• Levonorgestrelinasingledose(1.5mg)orasasplitdose
(1 dose of 0.75 mg of levonorgestrel followed by a second
dose of 0.75 mg of levonorgestrel 12 hours later)
• Combinedestrogenandprogestinin2doses(Yuzperegimen:
1 dose of 100 µg of ethinyl estradiol plus 0.50 mg of
levonorgestrel followed by a second dose of 100
µg of ethinyl
estradiol plus 0.50 mg of levonorgestrel 12 hours later)
Initiation of Emergency Contraception
Timing
Cu-IUD
• TheCu-IUDcanbeinsertedwithin5daysofthefirstactof
unprotected sexual intercourse as an emergency contraceptive.
• Inaddition,whenthedayofovulationcanbeestimated,
the Cu-IUD can be inserted beyond 5 days after sexual
intercourse, as long as insertion does not occur >5 days
after ovulation.
ECPs
• ECPsshouldbetakenassoonaspossiblewithin5daysof
unprotected sexual intercourse.
Comments and Evidence Summary. Cu-IUDs are highly
effective as emergency contraception (256) and can be
continued as regular contraception. UPA and levonorgestrel
ECPs have similar effectiveness when taken within 3 days after
unprotected sexual intercourse; however, UPA has been shown
to be more effective than the levonorgestrel formulation 3–5
days after unprotected sexual intercourse (257). The combined
estrogen and progestin regimen is less effective than UPA
or levonorgestrel and also is associated with more frequent
occurrence of side effects (nausea and vomiting) (258). The
levonorgestrel formulation might be less effective than UPA
among obese women (257).
Two studies of UPA use found consistent decreases in
pregnancy rates when administered within 120 hours of
unprotected sexual intercourse (257,259). Five studies found
that the levonorgestrel and combined regimens decreased risk
for pregnancy through the fifth day after unprotected sexual
intercourse; however, rates of pregnancy were slightly higher
when ECPs were taken after 3 days (260–264). A meta-analysis
of levonorgestrel ECPs found that pregnancy rates were low
when administered within 4 days after unprotected sexual
intercourse but increased at 4–5 days (265) (Level of evidence:
I to II-2, good to poor, direct).
Advance Provision of ECPs
• AnadvancesupplyofECPsmaybeprovidedsothatECPs
will be available when needed and can be taken as soon as
possible after unprotected sexual intercourse.
Comments and Evidence Summary. A systematic review
identified 17 studies that reported on safety or effectiveness of
advance ECPs in adult or adolescent women (26). Any use of
ECPs was two to seven times greater among women who received
an advance supply of ECPs. However, a summary estimate
(relative risk = 0.97; 95% confidence interval = 0.77–1.22) of
five randomized controlled trials did not indicate a significant
reduction in unintended pregnancies at 12 months with
advance provision of ECPs. In the majority of studies among
adults or adolescents, patterns of regular contraceptive use,
pregnancy rates, and incidence of STDs did not vary between
those who received advance ECPs and those who did not.
Although available evidence supports the safety of advance
Early Release
MMWR / June 14, 2013 / Vol. 62 35
provision of ECPs, effectiveness of advance provision of ECPs
in reducing pregnancy rates at the population level has not been
demonstrated (Level of evidence: I to II-3, good to poor, direct).
Initiation of Regular Contraception
After ECPs
UPA
• Any regular contraceptive method can be started
immediately after the use of UPA.
• Thewomanneedstoabstainfromsexualintercourseor
use barrier contraception for 14 days or until her next
menses, whichever comes first.
• Advisethewomantohaveapregnancytestifshedoesnot
have a withdrawal bleed within 3 weeks.
Levonorgestrel and Combined Estrogen and
Progestin ECPs
• Any regular contraceptive method can be started
immediately after the use of levonorgestrel or combined
estrogen and progestin ECPs.
• Thewomanneedstoabstainfromsexualintercourseor
use barrier contraception for 7 days.
• Advisethewomantohaveapregnancytestifshedoesnot
have a withdrawal bleed within 3 weeks.
Comments and Evidence Summary. Data on when a
woman can start regular contraception after ECPs are limited
to expert opinion and product labeling (27). Theoretically,
the effectiveness of systemic hormonal contraception might
be decreased when administered concurrently or in close
succession because of the antiprogestin properties of UPA
(266,267); these theoretical concerns do not exist for combined
estrogen and progestin or levonorgestrel formulations of
ECPs. The resumption or initiation of regular hormonal
contraception after ECP use involves consideration of the
risk for pregnancy if ECPs fail and the risks for unintended
pregnancy if contraception initiation is delayed until the
subsequent menstrual cycle. If a woman is planning to initiate
contraception after the next menstrual period after ECP
use, the cycle in which ECPs are used might be shortened,
prolonged, or involve unscheduled bleeding.
Prevention and Management of Nausea
and Vomiting with ECP Use
Nausea and Vomiting
• Levonorgestreland UPA ECPscause less nausea and
vomiting than combined estrogen and progestin ECPs.
• Routineuse ofantiemetics before taking ECPsis not
recommended. Pretreatment with antiemetics may be
considered depending on availability and clinical judgment.
Vomiting Within 3 Hours of Taking ECPs
• AnotherdoseofECPshouldbetakenassoonaspossible.
Use of an antiemetic should be considered.
Comments and Evidence Summary. Many women do
not experience nausea or vomiting when taking ECPs, and
predicting which women will experience nausea or vomiting
is difficult. Although routine use of antiemetics before taking
ECPs is not recommended, antiemetics are effective in some
women and can be offered when appropriate. Health-care
providers who are deciding whether to offer antiemetics to
women taking ECPs should consider the following: 1) women
taking combined estrogen and progestin ECPs are more
likely to experience nausea and vomiting than those who
take levonorgestrel or UPA ECPs; 2) evidence indicates that
antiemetics reduce the occurrence of nausea and vomiting in
women taking combined estrogen and progestin ECPs; and
3) women who take antiemetics might experience other side
effects from the antiemetics.
A systematic review examined incidence of nausea and
vomiting with different ECP regimens and effectiveness of
antinausea drugs in reducing nausea and vomiting with ECP
use (28). The levonorgestrel regimen was associated with
significantly less nausea than a nonstandard dose of UPA
(50 mg) and the standard combined estrogen and progestin
regimen (268–270). Use of the split-dose levonorgestrel
showed no differences in nausea and vomiting compared
with the single-dose levonorgestrel (260,261,263,271) (Level
of evidence: I, good-fair, indirect). Two trials of antinausea
drugs, meclizine and metoclopramide, taken before combined
estrogen and progestin ECPs, reduced the severity of nausea
(272,273). Significantly less vomiting occurred with meclizine
but not metoclopramide (Level of evidence: I, good-fair,
direct). No direct evidence was found regarding the effects of
vomiting after taking ECPs.
Female Sterilization
Laparoscopic, abdominal, and hysteroscopic methods of
female sterilization are available in the United States, and
some of these procedures can be performed in an outpatient
procedure or office setting. Fewer than 1 out of 100 women
become pregnant in the first year after female sterilization
(59). Because these methods are intended to be irreversible,
all women should be appropriately counseled about the
permanency of sterilization and the availability of highly
Early Release
36 MMWR / June 14, 2013 / Vol. 62
effective, long-acting, reversible methods of contraception.
Female sterilization does not protect against STDs; consistent
and correct use of male latex condoms reduces the risk for
STDs, including HIV.
When Hysteroscopic Sterilization Is
Reliable for Contraception
• Beforeawomancanrelyonhysteroscopicsterilizationfor
contraception, a hysterosalpingogram (HSG) must be
performed 3 months after the sterilization procedure to
confirm bilateral tubal occlusion.
• Thewomanshouldbeadvisedthatsheneedstoabstain
from sexual intercourse or use additional contraceptive
protection until she has confirmed bilateral tubal occlusion.
When Laparoscopic and Abdominal
Approches Are Reliable for Contraception
• A woman canrely on sterilization for contraception
immediately after laparoscopic and abdominal approaches.
No additional contraceptive protection is needed.
Comments and Evidence Summary. HSG confirmation
is necessary to confirm bilateral tubal occlusion after
hysteroscopic sterilization. The inserts for the hysteroscopic
sterilization system available in the United States are placed
bilaterally into the fallopian tubes and require 3 months
for adequate fibrosis and scarring leading to bilateral tubal
occlusion. After hysteroscopic sterilization, advise the woman
to correctly and consistently use an effective method of
contraception while awaiting confirmation. If compliance
with another method might be a problem, a woman and her
health-care provider may consider DMPA injection at the time
of sterilization to ensure adequate contraception for 3 months.
Unlike laparoscopic and abdominal sterilizations, pregnancy
risk beyond 7 years of follow-up has not been studied among
women who received hysteroscopic sterilization.
Pregnancy risk with at least 10 years of follow-up has
been studied among women who received laparoscopic and
abdominal sterilizations (274,275). Although these methods
are highly effective, pregnancies can occur many years after
the procedure, and the risk for pregnancy is higher among
younger women (274,276).
A systematic review was conducted to identify studies that
reported whether pregnancies occurred after hysteroscopic
sterilization (29). Twenty-four studies were identified that
reported whether pregnancies occurred after hysteroscopic
sterilization and found that very few pregnancies occurred
among women with confirmed bilateral tubal occlusion;
however, few studies include long-term follow-up, and
none with follow-up for >7 years. Among women who had
successful bilateral placement, most pregnancies that occurred
after hysteroscopic sterilization were in women who did
not have confirmed bilateral tubal occlusion at 3 months,
either because of lack of follow up or misinterpretation of
HSG results (277–279). Some pregnancies occurred within
3 months of placement, including among women who were
already pregnant at the time of the procedure, women who
did not use alternative contraception, or women who had
failures of alternative contraception (277,278,280–283).
Although these studies generally demonstrated high rates of
bilateral placement, some pregnancies occurred as a result
of lack of bilateral placement identified on later imaging
(277,278,280,281,283,284). Most pregnancies occurred after
deviations from FDA directions, which include placement in
the early follicular phase of the menstrual cycle, imaging at
3 months to document proper placement, and use of effective
alternative contraception until documented occlusion (Level
of evidence: II-3, fair, direct).
Male Sterilization
Male sterilization, or vasectomy, is one of the few
contraceptive methods available to men and can be performed
in an outpatient procedure or office setting. Fewer than 1
woman out of 100 becomes pregnant in the first year after
her male partner undergoes sterilization (59). Because male
sterilization is intended to be irreversible, all men should be
appropriately counseled about the permanency of sterilization
and the availability of highly effective, long-acting, reversible
methods of contraception for women. Male sterilization does
not protect against STDs; consistent and correct use of male
latex condoms reduces the risk for STDs, including HIV.
When Vasectomy Is Reliable for
Contraception
• Asemenanalysisshouldbeperformed8–16weeksafter
a vasectomy to ensure the procedure was successful.
• Themanshouldbeadvisedthatheshoulduseadditional
contraceptive protection or abstain from sexual intercourse
until he has confirmation of vasectomy success by
postvasectomy semen analysis.
Other Postprocedure Recommendations
• Themanshouldrefrainfromejaculationforapproximately
1 week after the vasectomy to allow for healing of surgical
sites and, after certain methods of vasectomy, occlusion
of the vas.
Early Release
MMWR / June 14, 2013 / Vol. 62 37
Comments and Evidence Summary. The Vasectomy
Guideline Panel of the American Urological Association
performed a systematic review of key issues concerning the
practice of vasectomy (285). All English-language publications
on vasectomy published during 1949–2011 were reviewed. For
more information, see the American Urological Association
Vasectomy Guidelines (available at http://www.auanet.org/
education/vasectomy.cfm).
Motile sperm disappear within a few weeks after vasectomy
(286–289). The time to azoospermia varies widely in different
studies; however, by 12 weeks after the vasectomy, 80% of men
have azoospermia, and almost all others have rare nonmotile
sperm (defined as ≤100,000 nonmotile sperm per milliliter)
(285). The number of ejaculations after vasectomy is not a
reliable indicator of when azoospermia or rare nonmotile sperm
will be achieved (285). Once azoospermia or rare nonmotile
sperm has been achieved, patients can rely on the vasectomy for
contraception, although not with 100% certainty. The risk for
pregnancy after a man has achieved postvasectomy azoospermia
is approximately one in 2,000 (290–294).
A median of 78% (range 33%–100%) of men return for
a single postvasectomy semen analysis (285). In the largest
cohorts that appear typical of North American vasectomy
practice, approximately two thirds of men (55%–71%) return
for at least one postvasectomy semen analysis (291,295–299).
Assigning men an appointment after their vasectomy might
improve compliance with follow-up (300).
When Women Can Stop Using
Contraceptives
• Contraceptiveprotectionisstillneededforwomenaged
>44 years if the woman wants to avoid pregnancy.
Comments and Evidence Summary. The age at which
a woman is no longer at risk for pregnancy is not known.
Although uncommon, spontaneous pregnancies occur
among women aged >44 years. Both the American College
of Obstetricians and Gynecologists and the North American
Menopause Society recommend that women continue
contraceptive use until menopause or age 50–55 years
(301,302). The median age of menopause is approximately 51
years in North America (301) but can vary from ages 40 to 60
years (303). The median age of definitive loss of natural fertility
is 41 years but can range up to age 51 years (304,305). No
reliable laboratory tests are available to confirm definitive loss
of fertility in a woman. The assessment of follicle-stimulating
hormone levels to determine when a woman is no longer fertile
might not be accurate (301).
Health-care providers should consider the risks for becoming
pregnant in a woman of advanced reproductive age, as well as any
risks of continuing contraception until menopause. Pregnancies
among women of advanced reproductive age are at higher
risk for maternal complications, such as hemorrhage, venous
thromboembolism, and death, and fetal complications, such
as spontaneous abortion, stillbirth, and congenital anomalies
(306–308). Risks associated with continuing contraception,
in particular risks for acute cardiovascular events (venous
thromboembolism, myocardial infarction, or stroke) or breast
cancer, also are important to consider. U.S. MEC states that
on the basis of age alone, women aged >45 years can use POPs,
implants, the LNG-IUD, or the Cu-IUD (U.S. MEC 1) (5).
Women aged >45 years generally can use combined hormonal
contraceptives and DMPA (U.S. MEC 2) (5). However, women
in this age group might have chronic conditions or other risk
factors that might render use of hormonal contraceptive methods
unsafe; U.S. MEC might be helpful in guiding the safe use of
contraceptives in these women.
The incidence of venous thromboembolism was higher
among oral contraceptive users aged ≥45 years compared with
younger oral contraceptive users in two studies (309–311);
however, an interaction between hormonal contraception
and increased age compared with baseline risk was not
demonstrated (309,310) or was not examined (311). The
relative risk for myocardial infarction was higher among all
oral contraceptive users than in nonusers, although a trend of
increased relative risk with increasing age was not demonstrated
(312,313). No studies were found regarding the risk for stroke
in COC users aged ≥45 years (Level of evidence: II-2, good
to poor, direct).
A pooled analysis by the Collaborative Group on Hormonal
Factors and Breast Cancer in 1996 (314) found small increased
relative risks for breast cancer among women aged ≥45 years
whose last use of combined hormonal contraceptives was <5
years previously and for those whose last use was 5–9 years
previously. Seven more recent studies suggested small but
nonsignificant increased relative risks for breast carcinoma
in situ or breast cancer among women who had used oral
contraceptives or DMPA when they were aged ≥40 years
compared with those who had never used either method
(315–321) (Level of evidence: II-2, fair, direct).
Conclusion
Women, men, and couples have increasing numbers of safe
and effective choices for contraceptive methods, including LARC
methods such as IUDs and implants, to reduce the risk for
unintended pregnancy. However, with these expanded options
Early Release
38 MMWR / June 14, 2013 / Vol. 62
comes the need for evidence-based guidance to help health-care
providers offer quality family planning care to their patients,
including choosing the most appropriate contraceptive method
for individual circumstances and using that method correctly,
consistently, and continuously to maximize effectiveness.
Removing unnecessary barriers can help patients access and
successfully use contraceptive methods. Several medical barriers
to initiating and continuing contraceptive methods might exist,
such as unnecessary screening examinations and tests before
starting the method (e.g., a pelvic examination before initiation
of COCs), inability to receive the contraceptive on the same
day as the visit (e.g., waiting for test results that might not be
needed or waiting until the woman’s next menstrual period to
start use), and difficulty obtaining continued contraceptive
supplies (e.g., restrictions on number of pill packs dispensed
at one time). Removing unnecessary steps, such as providing
prophylactic antibiotics at the time of IUD insertion or requiring
unnecessary follow-up procedures, also can help patients access
and successfully use contraception.
Most women can start most contraceptive methods at
any time, and few examinations or tests, if any, are needed
before starting a contraceptive method. Routine follow-up
for most women includes assessment of her satisfaction with
the contraceptive method, concerns about method use, and
changes in health status or medications that could affect
medical eligibility for continued use of the method. Because
changes in bleeding patterns are one of the major reasons
for discontinuation of contraception, recommendations are
provided for the management of bleeding irregularities with
various contraceptive methods. In addition, because women
and health-care providers can be confused about the procedures
for missed pills and dosing errors with the contraceptive patch
and ring, the instructions are streamlined for easier use. ECPs
and emergency use of the Cu-IUD are important options for
women, and recommendations on using these methods, as
well as starting regular contraception after use of emergency
contraception, are provided. Male and female sterilization are
highly effective methods of contraception for men, women, and
couples who have completed childbearing; for men undergoing
vasectomy and women undergoing a hysteroscopic sterilization
procedure, additional contraceptive protection is needed until
the success of the procedure can be confirmed.
CDC is committed to working with partners at the federal,
national, and local levels to disseminate, implement, and
evaluate the recommendations in U.S. SPR so that the
information reaches health-care providers. Strategies for
dissemination and implementation include collaborating
with other federal agencies and professional and service
organizations to widely distribute the recommendations
through presentations, electronic distribution, newsletters, and
other publications; development of provider tools and job aids
to assist providers in implementing the new recommendations;
and training activities for students, as well as for continuing
education. CDC will conduct a survey of family planning
health-care providers before and after release of this report
to assess attitudes and practices related to contraceptive
use. Results from this survey will assist CDC in evaluating
the impact of these recommendations on the provision
of contraceptives in the United States. Finally, CDC will
continually monitor new scientific evidence and will update
these recommendations as warranted by new evidence. Updates
to the recommendations, as well as provider tools and other
resources, are available on the CDC U.S. SPR website (http://
www.cdc.gov/reproductivehealth/UnintendedPregnancy/
USSPR.htm).
Acknowledgment
This report is based, in part, on the work of the Promoting Family
Planning Team, Department of Reproductive Health and Research,
World Health Organization, and its development of the Selected
Practice Recommendations for Contraceptive Use.
References
1. Finer LB, Zolna MR. Unintended pregnancy in the United States:
incidence and disparities, 2006. Contraception 2011;84:478–85.
2. Gipson JD, Koenig MA, Hindin MJ. The effects of unintended
pregnancy on infant, child, and parental health: a review of the literature.
Stud Fam Plann 2008;39:18–38.
3. Trussell J. The cost of unintended pregnancy in the United States.
Contraception 2007;75:168–70.
4. Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in
the United States, 1994 and 2001. Perspect Sex Reprod Health
2006;38:90–6.
5. CDC. U.S. medical eligibility criteria for contraceptive use. MMWR
2010;59(No. RR-4).
6. World Health Organization. Selected practice recommendations for
contraceptive use. Second ed. Geneva: World Health Organization; 2004.
7. World Health Organization. Selected practice recommendations for
contraceptive use. 2008 update. Geneva, Switzerland: World Health
Organization; 2008. Available at http://whqlibdoc.who.int/hq/2008/
WHO_RHR_08.17_eng.pdf.
8. Faculty of Sexual and Reproductive Health Care. UK Selected Practice
Recommendations for Contraceptive Use. London, England: Faculty of
Sexual and Reproductive Health Care; 2002. Available at http://www.
fsrh.org/pdfs/SelectedPracticeRecommendations2002.pdf.
9. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items
for systematic reviews and meta-analyses: the PRISMA statement. Int J
Surg 2010;8:336–41.
10. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational
studies in epidemiology: a proposal for reporting. Meta-analysis Of
Observational Studies in Epidemiology (MOOSE) group. JAMA
2000;283:2008–12.
11. Harris RP, Helfand M, Woolf SH, et al. Current methods of the US
Preventive Services Task Force: a review of the process. Am J Prev Med
2001;20(Suppl):21–35.
12. Tepper NK, Marchbanks PA, Curtis KM. Use of a checklist to rule out
pregnancy: a systematic review. Contraception 2013;87:661–5.
Early Release
MMWR / June 14, 2013 / Vol. 62 39
13. Whiteman MK, Tyler CP, Folger SG, Gaffield ME, Curtis KM. When
can a woman have an intrauterine device inserted? A systematic review.
Contraception 2013;87:666–73.
14. Tepper NK, Steenland MW, Marchbanks PA, Curtis KM. Laboratory
screening prior to initiating contraception: a systematic review.
Contraception 2013;87:645–9.
15. Tepper NK, Curtis KM, Steenland MW, Marchbanks PA. Physical
examination prior to initiating hormonal contraception: a systematic
review. Contraception 2013;87:650–4.
16. Steenland MW, Zapata LB, Brahmi D, Marchbanks PA, Curtis KM. The
effect of follow-up visits or contacts after contraceptive initiation on
method continuation and correct use. Contraception 2013;87:625–30.
17. Steenland MW, Zapata LB, Brahmi D, Marchbanks PA, Curtis KM.
Appropriate follow up to detect potential adverse events after initiation
of select contraceptive methods: a systematic review. Contraception
2013;87:611–24.
18. Godfrey EM, Folger SG, Jeng G, Jamieson DJ, Curtis KM. Treatment
of bleeding irregularities in women with copper-containing IUDs: a
systematic review. Contraception 2013;87:549–66.
19. Tepper NK, Steenland MW, Gaffield ME, Marchbanks PA, Curtis KM.
Retention of intrauterine devices in women who acquire pelvic inflammatory
disease: a systematic review. Contraception 2013;87:655–60.
20. Kapp N, Gaffield ME. Initiation of progestogen-only injectables on
different days of the menstrual cycle and its effect on contraceptive
effectiveness and compliance: a systematic review. Contraception
2013;87:576–82.
21. Tepper NK, Curtis KM, Steenland MW, Marchbanks PA. Blood pressure
measurement prior to initiating hormonal contraception: a systematic
review. Contraception 2013;87:631–8.
22. Brahmi D, Curtis KM. When can a woman start combined hormonal
contraceptives (CHCs)? A systematic review. Contraception 2013;
87:524–38.
23. Steenland MW, Rodriguez MI, Marchbanks PA, Curtis KM. How does
the number of oral contraceptive pill packs dispensed or prescribed affect
continuation and other measures of consistent and correct use? A
systematic review. Contraception 2013;87:605–10.
24. Zapata LB, Steenland MW, Brahmi D, Marchbanks PA, Curtis KM.
Effect of missed combined hormonal contraceptives on contraceptive
effectiveness: a systematic review. Contraception 2013;87:685–700.
25. Godfrey EM, Whiteman MK, Curtis KM. Treatment of unscheduled
bleeding in women using extended- or continuous-use combined hormonal
contraception: a systematic review. Contraception 2013;87:567–75.
26. Rodriguez MI, Curtis KM, Gaffield ML, Jackson E, Kapp N. Advance
supply of emergency contraception: a systematic review. Contraception
2013;87:590–601.
27. Salcedo J, Rodriguez MI, Curtis KM, Kapp N. When can a woman
resume or initiate contraception after taking emergency contraceptive
pills? A systematic review. Contraception 2013;87:602–4.
28. Rodriguez MI, Godfrey EM, Warden M, Curtis KM. Prevention and
management of nausea and vomiting with emergency contraception: a
systematic review. Contraception 2013;87:583–9.
29. Cleary TP, Tepper NK, Cwiak C, et al. Pregnancies after hysteroscopic
sterilization: a systematic review. Contraception 2013;87:539–48.
30. Tepper NK, Steenland MW, Marchbanks PA, Curtis KM. Hemoglobin
measurement prior to initiating copper intrauterine devices: a systematic
review. Contraception 2013;87:639–44.
31. Folger SG, Jamieson DJ, Godfrey EM, Zapata LB, Curtis KM. Evidence-
based guidance on selected practice recommendations for contraceptive
use: identification of research gaps. Contraception 2013;87:517–23.
32. CDC. Sexually transmitted diseases treatment guidelines. MMWR
2010;59(No. RR-12).
33. Mohllajee AP, Curtis KM, Flanagan RG, Rinehart W, Gaffield ML,
Peterson HB. Keeping up with evidence a new system for WHO’s evidence-
based family planning guidance. Am J Prev Med 2005;28:483–90.
34. Stanback J, Nakintu N, Qureshi Z, Nasution M. Does assessment of
signs and symptoms add to the predictive value of an algorithm to rule
out pregnancy? J Fam Plann Reprod Health Care 2006;32:27–9.
35.StanbackJ,NandaK,RamirezY,RountreeW,CameronSB.Validation
of a job aid to rule out pregnancy among family planning clients in
Nicaragua. Rev Panam Salud Publica 2008;23:116–8.
36. Stanback J, Qureshi Z, Sekadde-Kigondu C, Gonzalez B, Nutley T.
Checklist for ruling out pregnancy among family-planning clients in
primary care. Lancet 1999;354:566.
37. Torpey K, Mwenda L, Kabaso M, et al. Excluding pregnancy among
women initiating antiretroviral therapy: efficacy of a family planning
job aid. BMC Public Health 2010;10:249.
38. Cole LA, Ladner DG, Byrn FW. The normal variabilities of the menstrual
cycle. Fertil Steril 2009;91:522–7.
39. Wilcox AJ, Dunson D, Baird DD. The timing of the “fertile window”
in the menstrual cycle: day specific estimates from a prospective study.
BMJ 2000;321:1259–62.
40. Donnet ML, Howie PW, Marnie M, Cooper W, Lewis M. Return of
ovarian function following spontaneous abortion. Clin Endocrinol (Oxf)
1990;33:13–20.
41. Lahteenmaki P. Postabortal contraception. Ann Med 1993;25:185–9.
42. Stoddard A, Eisenberg DL. Controversies in family planning: timing of
ovulation after abortion and the conundrum of postabortion intrauterine
device insertion. Contraception 2011;84:119–21.
43. Jackson E, Glasier A. Return of ovulation and menses in postpartum, non-
lactating women: a systematic review. Obstet Gynecol 2011;117:657–62.
44. Kennedy KI, Rivera R, McNeilly AS. Consensus statement on the use of
breastfeeding as a family planning method. Contraception 1989;39:477–96.
45. Food and Drug Administration. 510(k) premarket notification. Silver
Spring, MD: Food and Drug Administration; 2013. Available at http://
www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm.
46. Cervinski MA, Gronowski AM. Reproductive-endocrine point-of-care testing:
current status and limitations. Clin Chem Lab Med 2010;48:935–42.
47. Cole LA. Human chorionic gonadotropin tests. Expert Rev Mol Diagn
2009;9:721–47.
48. Eichner SF, Timpe EM. Urinary-based ovulation and pregnancy: point-
of-care testing. Ann Pharmacother 2004;38:325–31.
49. Wilcox AJ, Baird DD, Dunson D, McChesney R, Weinberg CR. Natural
limits of pregnancy testing in relation to the expected menstrual period.
JAMA 2001;286:1759–61.
50.KorhonenJ, Alfthan H,Ylostalo P, Veldhuis J, StenmanUH.
Disappearance of human chorionic gonadotropin and its alpha- and
beta-subunits after term pregnancy. Clin Chem 1997;43:2155–63.
51. Reyes FI, Winter JS, Faiman C. Postpartum disappearance of chorionic
gonadotropin from the maternal and neonatal circulations. Am J Obstet
Gynecol 1985;153:486–9.
52. Steier JA, Bergsjo P, Myking OL. Human chorionic gonadotropin in
maternal plasma after induced abortion, spontaneous abortion, and
removed ectopic pregnancy. Obstet Gynecol 1984;64:391–4.
53. Bracken MB. Oral contraception and congenital malformations in
offspring: a review and meta-analysis of the prospective studies. Obstet
Gynecol 1990;76:552–7.
54. Gray RH, Pardthaisong T. In utero exposure to steroid contraceptives
and survival during infancy. Am J Epidemiol 1991;134:804–11.
55. Pardthaisong T, Gray RH. In utero exposure to steroid contraceptives
and outcome of pregnancy. Am J Epidemiol 1991;134:795–803.
56. Jaffe B, Harlap S, Baras M, et al. Long-term effects of MPA on human
progeny: intellectual development. Contraception 1988;37:607–19.
57.PardthaisongT,Yenchit C, Gray R.The long-term growth and
development of children exposed to Depo-Provera during pregnancy or
lactation. Contraception 1992;45:313–24.
58. Brahmi D, Steenland MW, Renner RM, Gaffield ME, Curtis KM.
Pregnancy outcomes with an IUD in situ: a systematic review.
Contraception 2012;85:131–9.
Early Release
40 MMWR / June 14, 2013 / Vol. 62
59. Trussell J. Contraceptive failure in the United States. Contraception
2011;83:397–404.
60. Labbok M, Perez A, Valdes V, et al. The Lactational Amenorrhea Method
(LAM): a postpartum introductory family planning method with policy
and program implications. Adv Contracept 1994;10:93–109.
61. Teva Women’s Health. ParaGard physicians prescribing information.
Sellersville, PA: Teva Women’s Health; 2013. Available at http://www.
paragard.com/images/ParaGard_info.pdf.
62. Bayer HealthCare Pharmaceuticals. Mirena physicians prescribing
information. Wayne, NJ: Bayer HealthCare Pharmaceuticals; 2013.
Available at http://www.berlex.com/html/products/pi/Mirena_PI.pdf.
63. Farley TM, Rosenberg MJ, Rowe PJ, Chen JH, Meirik O. Intrauterine
devices and pelvic inflammatory disease: an international perspective.
Lancet 1992;339:785–8.
64. Mohllajee AP, Curtis KM, Peterson HB. Does insertion and use of an
intrauterine device increase the risk of pelvic inflammatory disease among
women with sexually transmitted infection? A systematic review.
Contraception 2006;73:145–53.
65. Rivera R, Almonte H, Arreola M, et al. The effects of three different
regimens of oral contraceptives and three different intrauterine devices
on the levels of hemoglobin, serum iron and iron binding capacity in
anemic women. Contraception 1983;27:311–27.
66. Effects of contraceptives on hemoglobin and ferritin. Task Force for
Epidemiological Research on Reproductive Health, United Nations
Development Programme/United Nations Population Fund/World Health
Organization/World Bank Special Programme of Research, Development
and Research Training in Human Reproduction, World Health
Organization, Geneva, Switzerland. Contraception 1998;58:262–73.
67. Calzolari E, Guglielmo R, Viola F, Migliore L. Hematological parameters
and iron therapy in women with IUDs: experimental study. Minerva
Ginecol 1981;33:355–62.
68. Hassan EO, El-Husseini M, El-Nahal N. The effect of 1-year use of the
CuT 380A and oral contraceptive pills on hemoglobin and ferritin levels.
Contraception 1999;60:101–5.
69. Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-
releasing (Nova T) IUDs during five years of use: a randomized
comparative trial. Contraception 1994;49:56–72.
70. Andrade AT, Pizarro E, Shaw STJ, Souza JP, Belsey EM, Rowe PJ.
Consequences of uterine blood loss caused by various intrauterine
contraceptive devices in South American women. World Health
Organization Special Programme of Research, Development and Research
Training in Human Reproduction. Contraception 1988;38:1–18.
71. Blum M, Ariel J, Zacharowitch D. Ferritin, a faithful reflection of iron
deficiency in IUD wearers with mild vaginal spotting. Adv Contracept
1991;7:39–42.
72. El-sheikha Z, Hamza A, Mahmoud M. Menstrual blood loss of TCu-380
A and TCu-200 B IUDs. Popul Sci 1990;9:55–62.
73. Gallegost AJ, Aznar R, Merino G, Guizer E. Intrauterine devices and
menstrual blood loss: a comparative study of eight devices during the
first six months of use. Contraception 1978;17:153–61.
74. Gao J, Zeng S, Sun BL, et al. Menstrual blood loss, haemoglobin and
ferritin concentration of Beijing women wearing steel ring, VCu 200,
and TCu 220c IUDs. Contraception 1986;34:559–71.
75. Goh TH, Hariharan M. Effect of laparoscopic sterilization and insertion
of Multiload Cu 250 and Progestasert IUDs on serum ferritin levels.
Contraception 1983;28:329–36.
76. Goh TH, Hariharan M, Tan CH. A longitudinal study of serum iron
indices and haemoglobin concentration following copper-IUD insertion.
Contraception 1980;22:389–95.
77. Guillebaud J, Bonnar J, Morehead J, Matthews A. Menstrual blood-loss
with intrauterine devices. Lancet 1976;1:387–90.
78. Haugan T, Skjeldestad FE, Halvorsen LE, Kahn H. A randomized trial
on the clinical performance of Nova T380 and Gyne T380 Slimline
copper IUDs. Contraception 2007;75:171–6.
79. Kivijarvi A, Timonen H, Rajamaki A, Gronroos M. Iron deficiency in
women using modern copper intrauterine devices. Obstet Gynecol
1986;67:95–8.
80. Larsson B, Hamberger L, Rybo G. Influence of copper intrauterine
contraceptive devices (Cu-7-IUD) on the menstrual blood-loss. Acta
Obstet Gynecol Scand 1975;54:315–8.
81. Larsson G, Milsom I, Jonasson K, Lindstedt G, Rybo G. The long-term
effects of copper surface area on menstrual blood loss and iron status in
women fitted with an IUD. Contraception 1993;48:471–80.
82. Malmqvist R, Petersohn L, Bengtsson LP. Menstrual bleeding with
copper-covered intrauterine contraceptive devices. Contraception
1974;9:627–33.
83. Milsom I, Andersson K, Jonasson K, Lindstedt G, Rybo G. The influence
of the Gyne-T 380S IUD on menstrual blood loss and iron status.
Contraception 1995;52:175–9.
84. Milsom I, Rybo G, Lindstedt G. The influence of copper surface area
on menstrual blood loss and iron status in women fitted with an IUD.
Contraception 1990;41:271–81.
85. Piedras J, Cordova MS, Perez-Toral MC, Lince E, Garza-Flores J.
Predictive value of serum ferritin in anemia development after insertion
of T Cu 220 intrauterine device. Contraception 1983;27:289–97.
86. Sivin I, Alvarez F, Diaz J, et al. Intrauterine contraception with copper
and with levonorgestrel: a randomized study of the TCu 380Ag and
levonorgestrel 20 mcg/day devices. Contraception 1984;30:443–56.
87. Sivin I. Two years of intrauterine contraception with levonorgestrel and
with copper: a randomized comparison of the TCu 380Ag and
levonorgestrel 20 mcg/day devices. Contraception 1987;35:245–55.
88. Tchai BS, Kim SW, Han JH, Im MW. Menstrual blood loss, iron
nutriture, and the effects of Alza-T IPCS 52, T-Cu 220C and Lippes
Loop D in Korean women. Seoul J Med 1987;28:51–9.
89. Wright EA, Kapu MM, Isichei UP. Zinc depletion and menorrhagia in
Nigerians using copper T-200 intrauterine device. Trace Elem Med
1989;6:147–9.
90. Pleis JR, Lucas JW, Ward BW. Summary health statistics for U.S. adults:
National Health Interview Survey, 2008. Vital Health Stat 10
2009;10:1–157.
91. CDC. Viral hepatitis surveillance, United States, 2009. Atlanta, GA: CDC;
2011. Available at http://www.cdc.gov/hepatitis/Statistics/2009Surveillance/
PDFs/2009HepSurveillanceRpt.pdf.
92. Kohler BA, Ward E, McCarthy BJ, et al. Annual report to the nation
on the status of cancer, 1975–2007, featuring tumors of the brain and
other nervous system. J Natl Cancer Inst 2011;103:714–36.
93. Kapp N, Curtis KM. Hormonal contraceptive use among women with
liver tumors: a systematic review. Contraception 2009;80:387–90.
94. Kapp N, Tilley IB, Curtis KM. The effects of hormonal contraceptive
use among women with viral hepatitis or cirrhosis of the liver: a
systematic review. Contraception 2009;80:381–6.
95. National Cancer Institute. National Cancer Institute at the National
Institutes for Health [homepage]. Bethesda, MD: National Cancer
Institute. Available at http://www.cancer.gov.
96. Watson M, Saraiya M, Benard V, et al. Burden of cervical cancer in the
United States, 1998–2003. Cancer 2008;113(Suppl):2855–64.
97. CDC. Cervical cancer screening among women aged 18-30 years—
United States, 2000–2010. MMWR 2013;61:1038–42.
98. Curtis KM, Nanda K, Kapp N. Safety of hormonal and intrauterine
methods of contraception for women with HIV/AIDS: a systematic
review. AIDS 2009;23(Suppl 1):S55–67.
99. Grimes DA, Schulz KF. Prophylactic antibiotics for intrauterine device
insertion: a metaanalysis of the randomized controlled trials.
Contraception 1999;60:57–63.
Early Release
MMWR / June 14, 2013 / Vol. 62 41
100. Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective
endocarditis: guidelines from the American Heart Association: a
guideline from the American Heart Association Rheumatic Fever,
Endocarditis, and Kawasaki Disease Committee, Council on
Cardiovascular Diseasein theYoung, and the Council on Clinical
Cardiology, Council on Cardiovascular Surgery and Anesthesia, and
the Quality of Care and Outcomes Research Interdisciplinary Working
Group. Circulation 2007;116:1736–54.
101. Canto De Cetina TE, Canto P, Ordonez Luna M. Effect of counseling
to improve compliance in Mexican women receiving depot-
medroxyprogesterone acetate. Contraception 2001;63:143–6.
102. Lei ZW, Wu SC, Garceau RJ, et al. Effect of pretreatment counseling on
discontinuation rates in Chinese women given depo-medroxyprogesterone
acetate for contraception. Contraception 1996;53:357–61.
103. Toppozada M, Anwar M, Abdel Rahman H, Gaweesh S. Control of
IUD-induced bleeding by three non-steroidal anti-inflammatory drugs.
Contracept Deliv Syst 1982;3:117–25.
104.ToppozadaM,El-AttarA,El-AyyatMA,KhamisY.Managementof
uterine bleeding by PGs or their synthesis inhibitors. Adv Prostaglandin
Thromboxane Res 1980;8:1459–63.
105. Wu S, Wang C, Cheng W, et al. Randomized multi-center study of
baofuxin for treatment of bleeding side-effect induced by IUD. Reprod
Contracept 2000;11:152–7.
106. Mercorio F, De Simone R, Di Carlo C, et al. Effectiveness and mechanism
of action of desmopressin in the treatment of copper intrauterine device-
related menorrhagia: a pilot study. Hum Reprod 2003;18:2319–22.
107. Pedron N, Lozano M, Aznar R. Treatment of hypermenorrhea with mefenamic
acid in women using IUDs. Contracept Deliv Syst 1982;3:135–9.
108. Pizarro E, Mehech G, Hidalgo M, Munoz G, Romero C. Effect of
meclofenamic acid on menstruation in hypermenorrheic women using
intrauterine devices. Rev Chil Obstet Ginecol 1988;53:43–56.
109. Chinese National IUD Research Working Group. Prevention and treatment
of IUD-induced menorrhagia with antifibrinolytic and antiprostaglandin
drugs. Zhonghua Fu Chan Ke Za Zhi 1987;22:291–4, 312.
110. Di Lieto A, Catalano D, Miranda L, Paladini A. Action of a prostaglandin
synthetase inhibitor on IUD associated uterine bleeding. Clin Exp Obstet
Gynecol 1987;14:41–4.
111.YlikorkalaO,Viinikka L. Comparison betweenantifibrinolytic and
antiprostaglandin treatment in the reduction of increased menstrual blood
loss in women with intrauterine contraceptive devices. Br J Obstet Gynaecol
1983;90:78–83.
112. Food and Drug Administration. FDA prescribing information. Cyklokapron:
tranexamicacidinjection.NewYork,NewYork;2011.Availableathttp://
www.accessdata.fda.gov/drugsatfda_docs/label/2011/019281s030lbl.pdf.
113. Food and Drug Administration. FDA prescribing information. Lysteda:
tranexamic acid tablets. Parsippany, New Jersey; 2011. Available at http://
www.accessdata.fda.gov/drugsatfda_docs/label/2011/022430s002lbl.pdf.
114. Pedron N, Lozano M, Gallegos AJ. The effect of acetylsalicylic acid on menstrual
blood loss in women with IUDs. Contraception 1987;36:295–303.
115. Hidalgo M, Bahamondes L, Perrotti M, Diaz J, Dantas-Monteiro C, Petta C.
Bleeding patterns and clinical performance of the levonorgestrel-releasing
intrauterine system (Mirena) up to two years. Contraception 2002;
65:129–32.
116. Larsson B, Wennergren M. Investigation of a copper-intrauterine device
(Cu-IUD) for possible effect on frequency and healing of pelvic
inflammatory disease. Contraception 1977;15:143–9.
117. Soderberg G, Lindgren S. Influence of an intrauterine device on the
course of an acute salpingitis. Contraception 1981;24:137–43.
118. Teisala K. Removal of an intrauterine device and the treatment of acute
pelvic inflammatory disease. Ann Med 1989;21:63–5.
119. Altunyurt S, Demir N, Posaci C. A randomized controlled trial of coil
removal prior to treatment of pelvic inflammatory disease. Eur J Obstet
Gynecol Reprod Biol 2003;107:81–4.
120. Stewart FH, Harper CC, Ellertson CE, Grimes DA, Sawaya GF, Trussell
J. Clinical breast and pelvic examination requirements for hormonal
contraception: current practice vs evidence. JAMA 2001;285:2232–9.
121. Mansour D, Korver T, Marintcheva-Petrova M, Fraser IS. The effects
of Implanon on menstrual bleeding patterns. Eur J Contracept Reprod
Health Care 2008;13(Suppl 1):13–28.
122. Abdel-Aleem H, D’Arcangues C, Vogelsong K, Gulmezoglu AM.
Treatment of vaginal bleeding irregularities induced by progestin only
contraceptives. Cochrane Database Syst Rev 2007;CD003449.
123. Archer DF, Philput CB, Levine AS, et al. Effects of ethinyl estradiol
and ibuprofen compared to placebo on endometrial bleeding, cervical
mucus and the postcoital test in levonorgestrel subcutaneous implant
users. Contraception 2008;78:106–12.
124. Buasang K, Taneepanichskul S. Efficacy of celecoxib on controlling irregular
uterine bleeding secondary to Jadelle use. J Med Assoc Thai 2009;92:301–7.
125. Phaliwong P, Taneepanichskul S. The effect of mefenamic acid on
controlling irregular uterine bleeding second to Implanon use. J Med Assoc
Thai 2004;87(Suppl 3):S64–8.
126. Weisberg E, Hickey M, Palmer D, et al. A randomized controlled trial
of treatment options for troublesome uterine bleeding in Implanon users.
Hum Reprod 2009;24:1852–61.
127. Weisberg E, Hickey M, Palmer D, et al. A pilot study to assess the effect of
three short-term treatments on frequent and/or prolonged bleeding compared
to placebo in women using Implanon. Hum Reprod 2006;21:295–302.
128. Cheng L, Zhu H, Wang A, Ren F, Chen J, Glasier A. Once a month
administration of mifepristone improves bleeding patterns in women using
subdermal contraceptive implants releasing levonorgestrel. Hum Reprod
2000;15:1969–72.
129. Alvarez-Sanchez F, Brache V, Thevenin F, Cochon L, Faundes A. Hormonal
treatment for bleeding irregularities in Norplant implant users. Am J Obstet
Gynecol 1996;174:919–22.
130. Diaz S, Croxatto HB, Pavez M, Belhadj H, Stern J, Sivin I. Clinical assessment
of treatments for prolonged bleeding in users of Norplant implants.
Contraception 1990;42:97–109.
131. Witjaksono J, Lau TM, Affandi B, Rogers PA. Oestrogen treatment for
increased bleeding in Norplant users: preliminary results. Hum Reprod
1996;11(Suppl 2):109–14.
132. Abdel-Aleem H, Shaaban OM, Amin AF, Abdel-Aleem AM. Tamoxifen
treatment of bleeding irregularities associated with Norplant use.
Contraception 2005;72:432–7.
133. Phupong V, Sophonsritsuk A, Taneepanichskul S. The effect of tranexamic
acid for treatment of irregular uterine bleeding secondary to Norplant use.
Contraception 2006;73:253–6.
134. D’Arcangues C, Piaggio G, Brache V, et al. Effectiveness and acceptability
of vitamin E and low-dose aspirin, alone or in combination, on Norplant-
induced prolonged bleeding. Contraception 2004;70:451–62.
135. Subakir SB, Setiadi E, Affandi B, Pringgoutomo S, Freisleben HJ.
Benefits of vitamin E supplementation to Norplant users–in vitro and
in vivo studies. Toxicology 2000;148:173–8.
136. Petta C, Faundes A, Dunson T, Ramos M, DeLucio M, Faundes D.
Timing of onset of contraceptive effectiveness in Depo-Provera users.
II. Effects on ovarian function. Fertil Steril 1998;70:817–20.
Early Release
42 MMWR / June 14, 2013 / Vol. 62
137. Petta C, Faundes A, Dunson T, Ramos M, DeLucio M, Faundes D.
Timing of onset of contraceptive effectiveness in Depo-Provera users:
Part I. Changes in cervical mucus. Fertil Steril 1998;69:252–7.
138. Siriwongse T, Snidvongs W, Tantayaporn P, Leepipatpaiboon S. Effect
of depo-medroxyprogesterone acetate on serum progesterone levels when
administered on various cycle days. Contraception 1982;26:487–93.
139. Balkus J, Miller L. Same-day administration of depot-medroxyprogesterone
acetate injection: a retrospective chart review. Contraception 2005;
71:395–8.
140. Morroni C, Grams M, Tiezzi L, Westhoff C. Immediate monthly combination
contraception to facilitate initiation of the depot medroxyprogesterone acetate
contraceptive injection. Contraception 2004;70:19–23.
141. Nelson A, Katz T. Initiation and continuation rates seen in 2-year experience
with Same Day injections of DMPA. Contraception 2007;75:84–7.
142. Rickert V, Tiezzi L, Lipshutz J, Leon J, Vaughan R, Westhoff C. Depo
Now: preventing unintended pregnancies among adolescents and young
adults. J Adolesc Health 2007;40:22–8.
143. Sneed R, Westhoff C, Morroni C, Tiezzi L. A prospective study of
immediate initiation of depo medroxyprogesterone acetate contraceptive
injection. Contraception 2005;71:99–103.
144. Cox S, Dietz P, Bowman B, Posner S. Prevalence of chronic conditions among
women of reproductive age. Third National Summit on Preconception
Health and Health Care; Tampa, FL.
145. Diab KM, Zaki MM. Contraception in diabetic women: comparative
metabolic study of Norplant, depot medroxyprogesterone acetate, low
dose oral contraceptive pill and CuT380A. J Obstet Gynaecol Res
2000;26:17–26.
146. Garg SK, Chase HP, Marshall G, Hoops SL, Holmes DL, Jackson WE.
Oral contraceptives and renal and retinal complications in young women
with insulin-dependent diabetes mellitus. JAMA 1994;271:1099–102.
147. Grigoryan OR, Grodnitskaya EE, Andreeva EN, Chebotnikova TV,
Melnichenko GA. Use of the NuvaRing hormone-releasing system in
late reproductive-age women with type 1 diabetes mellitus. Gynecol
Endocrinol 2008;24:99–104.
148. Kahn HS, Curtis KM, Marchbanks PA. Effects of injectable or
implantable progestin-only contraceptives on insulin-glucose metabolism
and diabetes risk. Diabetes Care 2003;26:216–25.
149. Lopez LM, Grimes DA, Schulz KF. Steroidal contraceptives: effect on
carbohydrate metabolism in women without diabetes mellitus.
Cochrane Database Syst Rev 2009;CD006133.
150. Rogovskaya S, Rivera R, Grimes DA, et al. Effect of a levonorgestrel
intrauterine system on women with type 1 diabetes: a randomized trial.
Obstet Gynecol 2005;105:811–5.
151. Troisi RJ, Cowie CC, Harris MI. Oral contraceptive use and glucose
metabolism in a national sample of women in the united states. Am J
Obstet Gynecol 2000;183:389–95.
152. Bonny A, Secic M, Cromer B. Early weight gain related to later weight
gain in adolescents on depot medroxyprogesterone acetate. Obstet Gynecol
2011;117:793–7.
153.LeY,RahmanM,BerensonA.Earlyweightgainpredictinglaterweight
gain among depot medroxyprogesterone acetate users. Obstet Gynecol
2009;114:279–84.
154. Risser WL. Weight change in adolescents who used hormonal contraception.
J Adolesc Health 1999;24:433–6.
155. Paulen ME, Curtis KM. When can a woman have repeat progestogen-
only injectables—depot medroxyprogesterone acetate or norethisterone
enantate? Contraception 2009;80:391–408.
156. Pardthaisong T. Return of fertility after use of the injectable contraceptive
Depo Provera: up-dated data analysis. J Biosoc Sci 1984;16:23–34.
157. Schwallie PC, Assenzo JR. The effect of depo-medroxyprogesterone acetate
on pituitary and ovarian function, and the return of fertility following its
discontinuation: a review. Contraception 1974;10:181–202.
158. Steiner MJ, Kwok C, Stanback J, et al. Injectable contraception: what should
the longest interval be for reinjections? Contraception 2008;77:410–4.
159. Bassol S, Garza-Flores J, Cravioto MC, et al. Ovarian function following
a single administration of depo-medroxyprogesterone acetate (DMPA)
at different doses. Fertil Steril 1984;42:216–22.
160. Fotherby K, Koetsawang S, Mathrubutham M. Pharmacokinetic study
of different doses of Depo Provera. Contraception 1980;22:527–36.
161. Fotherby K, Saxena BN, Shrimanker K, et al. A preliminary pharmacokinetic
and pharmacodynamic evaluation of depot-medroxyprogesterone acetate
and norethisterone oenanthate. Fertil Steril 1980;34:131–9.
162. Garza-Flores J, Cardenas S, Rodriguez V, Cravioto MC, Diaz-Sanchez V,
Perez-Palacios G. Return to ovulation following the use of long-acting injectable
contraceptives: a comparative study. Contraception 1985;31:361–6.
163. Jain J, Dutton C, Nicosia A, Wajszczuk C, Bode FR, Mishell DR Jr.
Pharmacokinetics, ovulation suppression and return to ovulation
following a lower dose subcutaneous formulation of Depo-Provera.
Contraception 2004;70:11–8.
164. Lan PT, Aedo AR, Landgren BM, Johannisson E, Diczfalusy E. Return
of ovulation following a single injection of depo-medroxyprogesterone
acetate: a pharmacokinetic and pharmacodynamic study. Contraception
1984;29:1–18.
165. Ortiz A, Hirol M, Stanczyk FZ, Goebelsmann U, Mishell DR. Serum
medroxyprogesterone acetate (MPA) concentrations and ovarian function
following intramuscular injection of depo-MPA. J Clin Endocrinol Metab
1977;44:32–8.
166. Saxena BN, Dusitsin N, Tankeyoon M, Chaudhury RR. Return of
ovulation after the cessation of depot-medroxy progesterone acetate
treatment in Thai women. J Med Assoc Thai 1980;63:66–9.
167.Toh YC,JainJ, Rahnny MH, Bode FR, RossD. Suppression of
ovulation by a new subcutaneous depot medroxyprogesterone acetate
(104 mg/0.65 mL) contraceptive formulation in Asian women. Clin
Ther 2004;26:1845–54.
168. Hubacher D, Lopez L, Steiner MJ, Dorflinger L. Menstrual pattern changes
from levonorgestrel subdermal implants and DMPA: systematic review
and evidence-based comparisons. Contraception 2009;80:113–8.
169. Arias RD, Jain JK, Brucker C, Ross D, Ray A. Changes in bleeding
patterns with depot medroxyprogesterone acetate subcutaneous
injection 104 mg. Contraception 2006;74:234–8.
170. Dempsey A, Roca C, Westhoff C. Vaginal estrogen supplementation during
Depo-Provera initiation: a randomized controlled trial. Contraception
2010;82:250–5.
171. Harel Z, Biro F, Kollar L, Riggs S, Flanagan P, Vaz R. Supplementation
with vitamin C and/or vitamin B(6) in the prevention of Depo-Provera
side effects in adolescents. J Pediatr Adolesc Gynecol 2002;15:153–8.
172. Nathirojanakun P, Taneepanichskul S, Sappakitkumjorn N. Efficacy
of a selective COX-2 inhibitor for controlling irregular uterine bleeding
in DMPA users. Contraception 2006;73:584–7.
173. Tantiwattanakul P, Taneepanichskul S. Effect of mefenamic acid on
controlling irregular uterine bleeding in DMPA users. Contraception
2004;70:277–9.
Early Release
MMWR / June 14, 2013 / Vol. 62 43
174. Said S, Sadek W, Rocca M, et al. Clinical evaluation of the therapeutic
effectiveness of ethinyl oestradiol and oestrone sulphate on prolonged
bleeding in women using depot medroxyprogesterone acetate for
contraception. World Health Organization, Special Programme of Research,
Development and Research Training in Human Reproduction, Task Force
on Long-acting Systemic Agents for Fertility Regulation. Hum Reprod
1996;11(Suppl 2):1–13.
175. Sadeghi-Bazargani H, Ehdaeivand F, Arshi S, Eftekhar H, Sezavar H,
Amanati L. Low-dose oral contraceptive to re-induce menstrual bleeding
in amenorrheic women on DMPA treatment: a randomized clinical trial.
Med Sci Monit 2006;12:CR420–5.
176. CDC. Update to CDC’s U.S. Medical Eligibility Criteria for Contraceptive
Use, 2010: Revised recommendations for the use of contraceptive methods
during the postpartum period. MMWR 2011;60:878–83.
177. Westhoff C, Morroni C, Kerns J, Murphy PA. Bleeding patterns after
immediate vs. conventional oral contraceptive initiation: a randomized,
controlled trial. Fertil Steril 2003;79:322–9.
178. Westhoff C, Heartwell S, Edwards S, et al. Initiation of oral contraceptives
using a quick start compared with a conventional start—A randomized
controlled trial. Obstet Gynecol 2007;109:1270–6.
179. Edwards SM, Zieman M, Jones K, Diaz A, Robilotto C, Westhoff C. Initiation
of oral contraceptives—start now! J Adolesc Health 2008;43:432–6.
180. Baerwald AR, Olatunbosun OA, Pierson RA. Ovarian follicular development
is initiated during the hormone-free interval of oral contraceptive use.
Contraception 2004;70:371–7.
181. Baerwald AR, Pierson RA. Ovarian follicular development during the use
of oral contraception: a review. J Obstet Gynaecol Can 2004;26:19–24.
182. Duijkers IJM, Klipping C, Verhoeven CHJ, Dieben TOM. Ovarian
function with the contraceptive vaginal ring or an oral contraceptive:
a randomized study. Human Reproduction 2004;19):2668–73.
183. Killick S, Eyong E, Elstein M. Ovarian follicular development in oral
contraceptive cycles. Fertil Steril 1987;48:409–13.
184. Molloy BG, Coulson KA, Lee JM, Watters JK. “Missed pill” conception:
fact or fiction? Br Med J (Clin Res Ed) 1985;290:1474–5.
185. Mulders TM, Dieben TO, Bennink HJ. Ovarian function with a novel
combined contraceptive vaginal ring. Hum Reprod 2002;17:2594–9.
186. Schwartz JL, Creinin MD, Pymar HC, Reid L. Predicting risk of ovulation
in new start oral contraceptive users. Obstet Gynecol 2002;99:177–82.
187. Sitavarin S, Jaisamrarn U, Taneepanichskul S. A randomized trial on
the impact of starting day on ovarian follicular activity in very low dose
oral contraceptive pills users. J Med Assoc Thai 2003;86:442–8.
188. Smith SK, Kirkman RJ, Arce BB, McNeilly AS, Loudon NB, Baird DT.
The effect of deliberate omission of Trinordiol or Microgynon on the
hypothalamo-pituitary-ovarian axis. Contraception 1986;34:513–22.
189. Taylor DR, Anthony FW, Dennis KJ. Suppression of ovarian function by
Microgynon 30 in day 1 and day 5 “starters.” Contraception 1986;
33:463–71.
190. Lara-Torre E, Schroeder B. Adolescent compliance and side effects with Quick
Start initiation of oral contraceptive pills. Contraception 2002;66:81–5.
191. Murthy AS, Creinin MD, Harwood B, Schreiber CA. Same-day initiation
of the transdermal hormonal delivery system (contraceptive patch) versus
traditional initiation methods. Contraception 2005;72:333–6.
192. Westhoff C, Osborne L, Schafer J, Morroni C. Bleeding patterns after
immediate initiation of an oral compared with a vaginal hormonal
contraceptive. Obstet Gynecol 2005;106:89–96.
193.YeshayaA,OrvietoR,KaplanB,etal.Flexiblestartingschedulefororal
contraception: effect on the incidence of breakthrough bleeding and
compliance. Eur J Contracept Reprod Health Care 1998;3:121–3.
194.YeshayaA,OrvietoR,KauschanskyA,etal.Adelayedstartingschedule
of oral contraception: the effect on the incidence of breakthrough
bleeding and compliance in women. Eur J Contracept Reprod Health
Care 1996;1:263–5.
195. Westhoff C, Kerns J, Morroni C, Cushman LF, Tiezzi L, Murphy PA.
Quick start: novel oral contraceptive initiation method. Contraception
2002;66:141–5.
196. Acute myocardial infarction and combined oral contraceptives: results
of an international multicentre case-control study. WHO Collaborative
Study of Cardiovascular Disease and Steroid Hormone Contraception.
Lancet 1997;349:1202–9.
197. Dunn N, Thorogood M, Faragher B, et al. Oral contraceptives and
myocardial infarction: results of the MICA case-control study. BMJ
1999;318:1579–83.
198. Lewis MA, Heinemann LA, Spitzer WO, MacRae KD, Bruppacher R. The
use of oral contraceptives and the occurrence of acute myocardial infarction in
young women. Results from the Transnational Study on Oral Contraceptives
andtheHealthofYoungWomen.Contraception1997;56:129–40.
199. Ischaemic stroke and combined oral contraceptives: results of an international,
multicentre, case-control study. WHO Collaborative Study of Cardiovascular
Disease and Steroid Hormone Contraception. Lancet 1996;348:498–505.
200. Heinemann LA, Lewis MA, Spitzer WO, Thorogood M, Guggenmoos-
Holzmann I, Bruppacher R. Thromboembolic stroke in young women. A
European case-control study on oral contraceptives. Transnational Research
Group on Oral Contraceptives and the HealthofYoung Women.
Contraception 1998;57:29–37.
201. Haemorrhagic stroke, overall stroke risk, and combined oral contraceptives:
results of an international, multicentre, case-control study. WHO
Collaborative Study of Cardiovascular Disease and Steroid Hormone
Contraception. Lancet 1996;348:505–10.
202. Berenson AB, Rahman M, Wilkinson G. Effect of injectable and oral
contraceptives on serum lipids. Obstet Gynecol 2009;114:786–94.
203. Dilbaz B, Ozdegirmenci O, Caliskan E, Dilbaz S, Haberal A. Effect of
etonogestrel implant on serum lipids, liver function tests and hemoglobin
levels. Contraception 2010;81:510–4.
204. Nelson A. Combined oral contraceptives. In: Hatcher RA, Trussel J, Nelson
A, Cates W Jr, Stewart FH, Kowal D, eds. Contraceptive technology. 19th
ed.NewYork,NY:ArdentMedia,Inc;2007:193–270.
205. Mohllajee AP, Curtis KM, Martins SL, Peterson HB. Does use of hormonal
contraceptives among women with thrombogenic mutations increase their
risk of venous thromboembolism? A systematic review. Contraception
2006;73:166–78.
206. Wu O, Robertson L, Twaddle S, et al. Screening for thrombophilia in
high-risk situations: systematic review and cost-effectiveness analysis. The
Thrombosis: Risk and Economic Assessment of Thrombophilia Screening
(TREATS) study. Health technology assessment (Winchester, England)
2006;10:1–110.
207. Blickstein D, Blickstein I. Oral contraception and thrombophilia. Curr
Opin Obstet Gynecol 2007;19:370–6.
208. Wu O, Greer IA. Is screening for thrombophilia cost-effective? Curr
Opin Hematol 2007;14:500–3.
209. Foster D, Hulett D, Bradsberry M, Darney P, Policar M. Number of
oral contraceptive pill packages dispensed and subsequent unintended
pregnancies. Obstet Gynecol 2011;117:566–72.
210. Foster D, Parvataneni R, de Bocanegra H, Lewis C, Bradsberry M,
Darney P. Number of oral contraceptive pill packages dispensed, method
continuation, and costs. Obstet Gynecol 2006;108:1107–14.
Early Release
44 MMWR / June 14, 2013 / Vol. 62
211. White KO, Westhoff C. The effect of pack supply on oral contraceptive
pill continuation: a randomized controlled trial. Obstet Gynecol 2011;
118:615–22.
212. Chin Quee D, Cuthbertson C, Janowitz B. Over-the-counter pill
provision: evidence from Jamaica. Stud Fam Plann 2006;37:99–110.
213. Anttila L, Kunz M, Marr J. Bleeding pattern with drospirenone 3
mg+ethinyl estradiol 20 mcg 24/4 combined oral contraceptive compared
with desogestrel 150 mcg+ethinyl estradiol 20 mcg 21/7 combined oral
contraceptive. Contraception 2009;80:445–51.
214. Chowdhury V, Joshi UM, Gopalkrishna K, Betrabet S, Mehta S, Saxena
BN. ‘Escape’ ovulation in women due to the missing of low dose combination
oral contraceptive pills. Contraception 1980;22:241–7.
215. Christin-Maitre S, Serfaty D, Chabbert-Buffet N, Ochsenbein E, Chassard
D, Thomas JL. Comparison of a 24-day and a 21-day pill regimen for the
novel combined oral contraceptive, nomegestrol acetate and 17beta-estradiol
(NOMAC/E2): a double-blind, randomized study. Hum Reprod
2011;26:1338–47.
216. Creinin MD, Lippman JS, Eder SE, Godwin AJ, Olson W. The effect of
extending the pill-free interval on follicular activity: triphasic norgestimate/35
micro g ethinyl estradiol versus monophasic levonorgestrel/20 micro g
ethinyl estradiol. Contraception 2002;66:147–52.
217. Dinger J, Minh TD, Buttmann N, Bardenheuer K. Effectiveness of
oral contraceptive pills in a large U.S. cohort comparing progestogen
and regimen. Obstet Gynecol 2011;117:33–40.
218. Elomaa K, Lahteenmaki P. Ovulatory potential of preovulatory sized follicles
during oral contraceptive treatment. Contraception 1999;60:275–9.
219. Elomaa K, Rolland R, Brosens I, et al. Omitting the first oral contraceptive
pills of the cycle does not automatically lead to ovulation. Am J Obstet
Gynecol 1998;179:41–6.
220. Endrikat J, Wessel J, Rosenbaum P, Dusterberg B. Plasma concentrations
of endogenous hormones during one regular treatment cycle with a low-
dose oral contraceptive and during two cycles with deliberate omission
of two tablets. Gynecol Endocrinol 2004;18:318–26.
221. Hamilton CJ, Hoogland HJ. Longitudinal ultrasonographic study of the
ovarian suppressive activity of a low-dose triphasic oral contraceptive during
correct and incorrect pill intake. Am J Obstet Gynecol 1989;161:1159–62.
222. Hedon B, Cristol P, Plauchut A, et al. Ovarian consequences of the transient
interruption of combined oral contraceptives. Int J Fertil 1992;37:270–6.
223. Killick SR. Ovarian follicles during oral contraceptive cycles: their
potential for ovulation. Fertil Steril 1989;52:580–2.
224. Killick SR, Bancroft K, Oelbaum S, Morris J, Elstein M. Extending
the duration of the pill-free interval during combined oral contraception.
Adv Contracept 1990;6:33–40.
225. Klipping C, Duijkers I, Trummer D, Marr J. Suppression of ovarian
activity with a drospirenone-containing oral contraceptive in a 24/4
regimen. Contraception 2008;78:16–25.
226. Klipping C, Marr J. Effects of two combined oral contraceptives
containing ethinyl estradiol 20 microg combined with either
drospirenone or desogestrel on lipids, hemostatic parameters and
carbohydrate metabolism. Contraception 2005;71:409–16.
227. Landgren BM, Csemiczky G. The effect of follicular growth and luteal
function of “missing the pill.” A comparison between a monophasic and a
triphasic combined oral contraceptive. Contraception 1991;43:149–59.
228. Landgren BM, Diczfalusy E. Hormonal consequences of missing the pill
during the first two days of three consecutive artificial cycles. Contraception
1984;29:437–46.
229. Letterie GS. A regimen of oral contraceptives restricted to the periovulatory
period may permit folliculogenesis but inhibit ovulation. Contraception
1998;57:39–44.
230. Letterie GS, Chow GE. Effect of “missed” pills on oral contraceptive
effectiveness. Obstet Gynecol 1992;79:979–82.
231. Morris SE, Groom GV, Cameron ED, Buckingham MS, Everitt JM, Elstein
M. Studies on low dose oral contraceptives: plasma hormone changes in relation
to deliberate pill (‘Microgynon 30’) omission. Contraception 1979;20:61–9.
232. Nakajima ST, Archer DF, Ellman H. Efficacy and safety of a new 24-day
oral contraceptive regimen of norethindrone acetate 1 mg/ethinyl estradiol
20 micro g (Loestrin 24 Fe). Contraception 2007;75:16–22.
233. Nuttall ID, Elstein M, McCafferty E, Seth J, Cameron ED. The effect of
ethinyl estradiol 20 mcg and levonorgestrel 250 mcg on the pituitary-
ovarian function during normal tablet-taking and when tablets are missed.
Contraception 1982;26:121–35.
234. Pierson RA, Archer DF, Moreau M, Shangold GA, Fisher AC, Creasy
GW. Ortho Evra/Evra versus oral contraceptives: follicular development
and ovulation in normal cycles and after an intentional dosing error.
Fertil Steril 2003;80:34–42.
235. Rible RD, Taylor D, Wilson ML, Stanczyk FZ, Mishell DR Jr. Follicular
development in a 7-day versus 4-day hormone-free interval with an oral
contraceptive containing 20 mcg ethinyl estradiol and 1 mg norethindrone
acetate. Contraception 2009;79:182–8.
236. Schlaff WD, Lynch AM, Hughes HD, Cedars MI, Smith DL. Manipulation
of the pill-free interval in oral contraceptive pill users: the effect on follicular
suppression. Am J Obstet Gynecol 2004;190:943–51.
237. Spona J, Elstein M, Feichtinger W, et al. Shorter pill-free interval in combined
oral contraceptives decreases follicular development. Contraception
1996;54:71–7.
238. Sullivan H, Furniss H, Spona J, Elstein M. Effect of 21-day and 24-day
oral contraceptive regimens containing gestodene (60 microg) and ethinyl
estradiol (15 microg) on ovarian activity. Fertil Steril 1999;72:115–20.
239. Wang E, Shi S, Cekan SZ, Landgren BM, Diczfalusy E. Hormonal
consequences of “missing the pill.” Contraception 1982;26:545–66.
240. Willis SA, Kuehl TJ, Spiekerman AM, Sulak PJ. Greater inhibition of
the pituitary–ovarian axis in oral contraceptive regimens with a shortened
hormone-free interval. Contraception 2006;74:100–3.
241. Abrams LS, Skee DM, Natarajan J, et al. Pharmacokinetics of norelgestromin
and ethinyl estradiol delivered by a contraceptive patch (Ortho Evra/Evra)
under conditions of heat, humidity, and exercise. J Clin Pharmacol
2001;41:1301–9.
242. Ahrendt HJ, Nisand I, Bastianelli C, et al. Efficacy, acceptability and
tolerability of the combined contraceptive ring, NuvaRing, compared
with an oral contraceptive containing 30 microg of ethinyl estradiol
and 3 mg of drospirenone. Contraception 2006;74:451–7.
243. Bjarnadottir RI, Tuppurainen M, Killick SR. Comparison of cycle control
with a combined contraceptive vaginal ring and oral levonorgestrel/ethinyl
estradiol. Am J Obstet Gynecol 2002;186:389–95.
244. Brucker C, Karck U, Merkle E. Cycle control, tolerability, efficacy and
acceptability of the vaginal contraceptive ring, NuvaRing: results of
clinical experience in Germany. Eur J Contracept Reprod Health Care
2008;13:31–8.
245. Dieben TO, Roumen FJ, Apter D. Efficacy, cycle control, and user
acceptability of a novel combined contraceptive vaginal ring. Obstet
Gynecol 2002;100:585–93.
246. Mulders TM, Dieben TO. Use of the novel combined contraceptive vaginal
ring NuvaRing for ovulation inhibition. Fertil Steril 2001;75:865–70.
247. Guilbert E, Boroditsky R, Black A, et al. Canadian Consensus Guideline
on Continuous and Extended Hormonal Contraception, 2007. J Obstet
Gynaecol Can 2007;29(Suppl 2):S1–32.
Early Release
MMWR / June 14, 2013 / Vol. 62 45
248. Wiegratz I, Stahlberg S, Manthey T, et al. Effect of extended-cycle
regimen with an oral contraceptive containing 30 mcg ethinylestradiol
and 2 mg dienogest on bleeding patterns, safety, acceptance and
contraceptive efficacy. Contraception 2011;84:133–43.
249. Sulak PJ, Kuehl TJ, Coffee A, Willis S. Prospective analysis of occurrence
and management of breakthrough bleeding during an extended oral
contraceptive regimen. Am J Obstet Gynecol 2006;195:935–41.
250. Sulak PJ, Smith V, Coffee A, Witt I, Kuehl AL, Kuehl TJ. Frequency
and management of breakthrough bleeding with continuous use of the
transvaginal contraceptive ring: a randomized controlled trial. Obstet
Gynecol 2008;112:563–71.
251. Kaneshiro B, Edelman A, Carlson N, Morgan K, Nichols M, Jensen J.
Treatment of unscheduled bleeding in continuous oral contraceptive users
with doxycycline: a randomized controlled trial. Obstet Gynecol 2010;
115:1141–9.
252. McCann MF, Potter LS. Progestin-only oral contraception: a comprehensive
review. Contraception 1994;50:S1–195.
253. Arevalo M, Jennings V, Sinai I. Efficacy of a new method of family
planning: the Standard Days Method. Contraception 2002;65:333–8.
254. Arevalo M, Sinai I, Jennings V. A fixed formula to define the fertile
window of the menstrual cycle as the basis of a simple method of natural
family planning. Contraception 1999;60:357–60.
255. Wilcox AJ, Dunson DB, Weinberg CR, Trussell J, Baird DD. Likelihood
of conception with a single act of intercourse: providing benchmark rates for
assessment of post-coital contraceptives. Contraception 2001;63:211–5.
256. Cleland K, Zhu H, Goldstuck N, Cheng L, Trussell J. The efficacy of
intrauterine devices for emergency contraception: a systematic review
of 35 years of experience. Hum Reprod 2012;27:1994–2000.
257. Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus
levonorgestrel for emergency contraception: a randomised non-
inferiority trial and meta-analysis. Lancet 2010;375:555–62.
258. Raymond E, Taylor D, Trussell JMJS. Minimum effectiveness of the
levonorgestrel regimen of emergency contraception. Contraception
2004;69:79–81.
259. Fine P, Mathe H, Ginde S, Cullins V, Morfesis J, Gainer E. Ulipristal
acetate taken 48–120 hours after intercourse for emergency contraception.
Obstet Gynecol 2010;115:257–63.
260. Dada OA, Godfrey EM, Piaggio G, von Hertzen H. A randomized, double-
blind, noninferiority study to compare two regimens of levonorgestrel for
emergency contraception in Nigeria. Contraception 2010;82:373–8.
261. Ngai SW, Fan S, Li S, et al. A randomized trial to compare 24 h versus
12 h double dose regimen of levonorgestrel for emergency contraception.
Hum Reprod 2005;20:307–11.
262. Ellertson C, Evans M, Ferden S, et al. Extending the time limit for
startingtheYuzperegimenofemergencycontraceptionto120hours.
Obstet Gynecol 2003;101:1168–71.
263. Von Hertzen H, Piaggio G, Ding J, et al. Low dose mifepristone and
two regimens of levonorgestrel for emergency contraception: a WHO
multicentre randomised trial. Lancet 2002;360:1803–10.
264. Rodrigues I, Grou F, Joly J. Effectiveness of emergency contraceptive
pills between 72 and 120 hours after unprotected sexual intercourse.
Am J Obstet Gynecol 2001;184:531–7.
265. Piaggio G, Kapp N, von Hertzen H. Effect on pregnancy rates of the delay
in the administration of levonorgestrel for emergency contraception: a
combined analysis of four WHO trials. Contraception 2011;84:35–9.
266. Watson Pharma Inc. Prescribing Information for Ella. 2010. Morristown, NJ.
267. Emergency contraceptive pills. Medical and service delivery guidelines. 2012.
Available at http://www.cecinfo.org/custom-content/uploads/2013/03/
Medical-and-Service-Delivery-Guidelines-English-2012.pdf.
268. Creinin MD, Schlaff W, Archer DF, et al. Progesterone receptor
modulator for emergency contraception - A randomized controlled trial.
Obstet Gynecol 2006;108:1089–97.
269. Farajkhoda T, Khoshbin A, Enjezab B, Bokaei M, Zarchi MK. Assessment
of two emergency contraceptive regimens in Iran: levonorgestrel versus
theYuzpe.NigerJClinPract2009;12:450–2.
270. Ho PC, Kwan MSW. A prospective randomized comparison of
levonorgestrelwiththeYuzperegimenin post-coitalcontraception.
Hum Reprod 1993;8:389–92.
271. Arowojolu AO, Okewole IA, Adekunle AO. Comparative evaluation of
the effectiveness and safety of two regimens of levonorgestrel for emergency
contraception in Nigerians. Contraception 2002;66:269–73.
272. Raymond EG, Creinin MD, Barnhart KT, Lovvorn AE, Rountree RW,
Trussell J. Meclizine for prevention of nausea associated with use of emergency
contraceptive pills: a randomized trial. Obstet Gynecol 2000;95:271–7.
273. Ragan RE, Rock RW, Buck HW. Metoclopramide pretreatment attenuates
emergency contraceptive-associated nausea. Am J Obstet Gynecol
2003;188:330–3.
274. Peterson HB, Xia Z, Hughes JM, Wilcox LS, Tylor LR, Trussell J. The risk
of pregnancy after tubal sterilization: findings from the U.S. Collaborative
Review of Sterilization. Am J Obstet Gynecol 1996;174:1161–8, 8–70.
275. Peterson HB. Sterilization. Obstet Gynecol 2008;111:189–203.
276. Lawrie TA, Nardin JM, Kulier R, Boulvain M. Techniques for the
interruption of tubal patency for female sterilisation. Cochrane Database
Syst Rev 2011; (2):CD003034.
277. Veersema S, Vleugels MP, Moolenaar LM, Janssen CA, Brolmann HA.
Unintended pregnancies after Essure sterilization in the Netherlands.
Fertil Steril 2010;93:35–8.
278. Levy B, Levie MD, Childers ME. A summary of reported pregnancies after
hysteroscopic sterilization. J Minim Invasive Gynecol 2007;14:271–4.
279. Legendre G, Levaillant JM, Faivre E, Deffieux X, Gervaise A, Fernandez H.
3D ultrasound to assess the position of tubal sterilization microinserts. Hum
Reprod 2011;26:2683–9.
280. Duffy S, Marsh F, Rogerson L, et al. Female sterilisation: a cohort
controlled comparative study of ESSURE versus laparoscopic sterilisation.
BJOG 2005;112:1522–8.
281. Levie MD, Chudnoff SG. Prospective analysis of office-based hysteroscopic
sterilization. J Minim Invasive Gynecol 2006;13:98–101.
282. Arjona JE, Mino M, Cordon J, Povedano B, Pelegrin B, Castelo-Branco
C. Satisfaction and tolerance with office hysteroscopic tubal sterilization.
Fertil Steril 2008;90:1182–6.
283. Grosdemouge I, Engrand JB, Dhainault C, et al. La pratique francaise
de la pose des implants de sterilisation tubaire Essure. Gynecol Obstet
Fertil 2009;37:389–95.
284. Shavell VI, Abdallah ME, Diamond MP, Berman JM. Placement of a
permanent birth control device at a university medical center. J Reprod
Med 2009;54:218–22.
285. American Urological Association. Vasectomy guideline 2012. Available
at http://www.auanet.org/education/vasectomy.cfm.
286. Bedford J, Zelikovsky G. Viability of spermatozoa in the human
ejaculate after vasectomy. Fertil Steril 1979;32:460–3.
287. Edwards I. Earlier testing after vasectomy, based on the absence of
motile sperm. Fertil Steril 1993;59:431–6.
288. Jouannet P, David G. Evolution of the properties of semen immediately
following vasectomy. Fertil Steril 1978;29:435–41.
289. Labrecque M, Hays M, Chen-Mok M, Barone M, Sokal D. Frequency
and patterns of early recanalization after vasectomy. BMC Urol 2006;6:25.
290. Philp T, Guillebaud J, Budd D. Complications of vasectomy: review of
16,000 patients. Br J Urol 1984;56:745–8.
Early Release
46 MMWR / June 14, 2013 / Vol. 62
291. Alderman PM. The lurking sperm. A review of failures in 8879
vasectomies performed by one physician. JAMA 1988;259:3142–4.
292. Black T, Francome C. The evolution of the Marie Stopes electrocautery
no-scalpel vasectomy procedure. J Fam Plann Reprod Health Care
2002;28:137–8.
293. Davies AH, Sharp RJ, Cranston D, Mitchell RG. The long-term outcome
following “special clearance” after vasectomy. Br J Urol 1990;66:211–2.
294. Philp T, Guillebaud J, Budd D. Late failure of vasectomy after two
documented analyses showing azoospermic semen. Br Med J (Clin Res Ed)
1984;289:77–9.
295. Belker A, Sexter M, Sweitzer S, Raff M. The high rate of noncompliance
for post-vasectomy semen examination: medical and legal considerations.
J Urol 1990;144:284–6.
296. Chawla A, Bowles B, Zini A. Vasectomy follow-up: clinical significance
of rare nonmotile sperm in postoperative semen analysis. Urology
2004;64:1212–5.
297. Labrecque M, Bedard L, Laperriere L. Efficacy and complications
associated with vasectomies in two clinics in the Quebec region. Can
Fam Physician 1998;44:1860–6.
298. Labrecque M, Nazerali H, Mondor M, Fortin V, Nasution M.
Effectiveness and complications associated with 2 vasectomy occlusion
techniques. J Urol 2002;168:2495–8.
299. Maatman TJ, Aldrin L, Carothers GG. Patient noncompliance after
vasectomy. Fertil Steril 1997;68:552–5.
300. Dhar NB, Jones JS, Bhatt A, Babineau D. A prospective evaluation of
the impact of scheduled follow-up appointments with compliance rates
after vasectomy. BJU Int 2007;99:1094–7.
301. Hillard PJ, Berek JS, Barss VA, et al. Guidelines for women’s health care:
a resource manual. 3rd ed. Washington, DC: American College of
Obstetricians and Gynecologists; 2007.
302. The North American Menopause Society. Prescription hormonal
therapies. Menopause practice: a clinician’s guide. 4th ed. 2010.
303. Te Velde ER, Pearson PL. The variability of female reproductive ageing.
Hum Reprod Update 2002;8:141–54.
304. Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms
and clinical consequences. Endocr Rev 2009;30:465–93.
305. Wood JW. Fecundity and natural fertility in humans. Oxf Rev Reprod
Biol 1989;11:61–109.
306. Bateman BT, Simpson LL. Higher rate of stillbirth at the extremes of
reproductive age: a large nationwide sample of deliveries in the United
States. Am J Obstet Gynecol 2006;194:840–5.
307. Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related
mortality in the United States, 1998 to 2005. Obstet Gynecol 2010;
116:1302–9.
308. Balasch J, Gratacós E. Delayed childbearing: effects on fertility and the
outcome of pregnancy. Curr Opin Obstet Gynecol 2012;24:187–93.
309. Lidegaard O, Lokkegaard E, Svendsen AL, Agger C. Hormonal
contraception and risk of venous thromboembolism: national follow-up
study. BMJ 2009;339:b2890.
310. Lidegaard O, Nielsen LH, Skovlund CW, Skjeldestad FE, Lokkegaard
E. Risk of venous thromboembolism from use of oral contraceptives
containing different progestogens and oestrogen doses: Danish cohort
study, 2001–9. BMJ 2011;343:d6423.
311. Nightingale AL, Lawrenson RA, Simpson EL, Williams TJ, MacRae
KD, Farmer RD. The effects of age, body mass index, smoking and
general health on the risk of venous thromboembolism in users of
combined oral contraceptives. Eur J Contracept Reprod Health Care
2000;5:265–74.
312. Slone D, Shapiro S, Kaufman DW, Rosenberg L, Miettinen OS, Stolley
PD. Risk of myocardial infarction in relation to current and discontinued
use of oral contraceptives. N Engl J Med 1981;305:420–4.
313. Tanis BC, van den Bosch MA, Kemmeren JM, et al. Oral contraceptives
and the risk of myocardial infarction. N Engl J Med 2001;345:1787–93.
314. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer
and hormonal contraceptives: collaborative reanalysis of individual data
on 53 297 women with breast cancer and 100 239 women without breast
cancer from 54 epidemiological studies. Lancet 1996;347:1713–27.
315. Gill JK, Press MF, Patel AV, Bernstein L. Oral contraceptive use and
risk of breast carcinoma in situ (United States). Cancer Causes Control
2006;17:1155–62.
316. Kumle M, Weiderpass E, Braaten T, Persson I, Adami HO, Lund E. Use
of oral contraceptives and breast cancer risk: the Norwegian-Swedish
Women’s Lifestyle and Health Cohort Study. Cancer Epidemiol
Biomarkers Prev 2002;11:1375–81.
317. Marchbanks PA, McDonald JA, Wilson HG, et al. Oral contraceptives
and the risk of breast cancer. N Engl J Med 2002;346:2025–32.
318. Newcomb PA, Longnecker MP, Storer BE, et al. Recent oral contraceptive
use and risk of breast cancer (United States). Cancer Causes Control
1996;7:525–32.
319. Rosenberg L, Palmer JR, Rao RS, et al. Case-control study of oral contraceptive
use and risk of breast cancer. Am J Epidemiol 1996;143:25–37.
320.RosenbergL,ZhangY,CooganPF,StromBL,PalmerJR.Acase-control
study of oral contraceptive use and incident breast cancer. Am J Epidemiol
2009;169:473–9.
321. Shapiro S, Rosenberg L, Hoffman M, et al. Risk of breast cancer in relation
to the use of injectable progestogen contraceptives and combined estrogen/
progestogen contraceptives. Am J Epidemiol 2000;151:396–403.
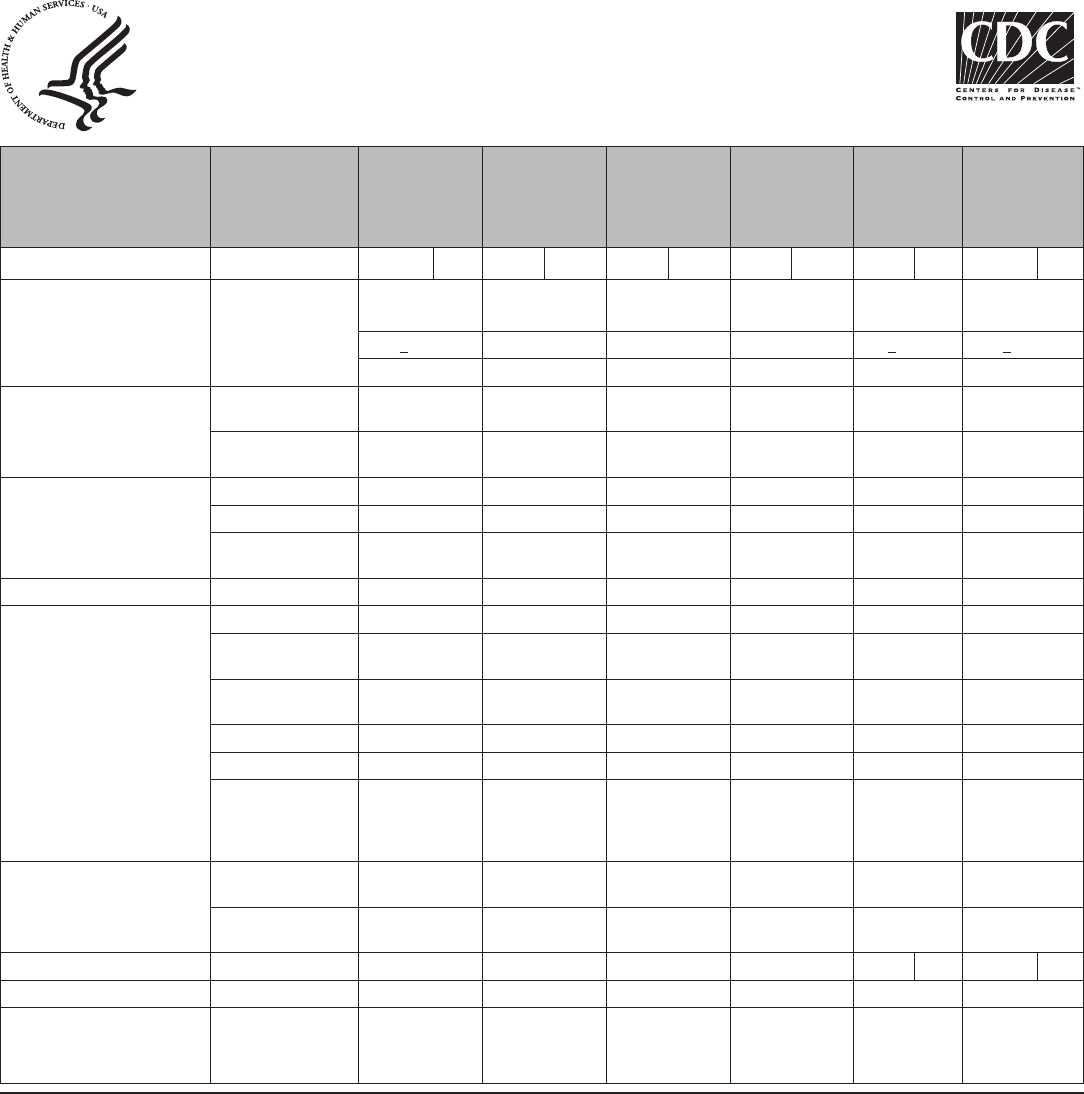
Early Release
MMWR / June 14, 2013 / Vol. 62 47
Appendix A
Summary Chart of U.S. Medical Eligibility Criteria for Contraceptive Use, 2010
Updated June 2012. This summary sheet only contains
a subset of the recommendations from the US MEC.
For complete guidance, see: http://www.cdc.gov/
reproductivehealth/unintendedpregnancy/USMEC.htm.
Most contraceptive methods do not protect against sexually
transmitted infections (STIs). Consistent and correct use of
the male latex condom reduces the risk of STIs and HIV.
Key:
1. No restriction (method can be used)
2. Advantages generally outweigh theoretical or proven
risks
3. Theoretical or proven risks usually outweigh the
advantages
4. Unacceptable health risk (method not to be used)
Condition Sub-condition
Combined
pill, patch,
ring
Progestin-
only pill Injection Implant LNG-IUD Copper-IUD
I C I C I C I C I C I C
Age Menarche to
<40=1
Menarche to
<18=1
Menarche to
<18=2
Menarche to
<18=1
Menarche to
<20=2
Menarche to
<20=2
>40=2 18-45=1 18-45=1 18-45=1 >20=1 >20=1
>45=1 >45=2 >45=1
Anatomic
abnormalities
a) Distorted uterine
cavity
4 4
b) Other
abnormalities
2 2
Anemias a) alassemia 1 1 1 1 1 2
b) Sickle cell disease
†
2 1 1 1 1 2
c) Iron-deciency
anemia
1 1 1 1 1 2
Benign ovarian tumors (including cysts) 1 1 1 1 1 1
Breast disease a) Undiagnosed mass 2* 2* 2* 2* 2 1
b) Benign breast
disease
1 1 1 1 1 1
c) Family history of
cancer
1 1 1 1 1 1
d) Breast cancer
†
i) current 4 4 4 4 4 1
ii) past and no
evidence of
current disease for
5 years
3 3 3 3 3 1
Breastfeeding
(see also Postpartum)
a) < 1 month
postpartum
3* 2* 2* 2*
b) 1 month or more
postpartum
2* 1* 1* 1*
Cervical cancer Awaiting treatment 2 1 2 2 4 2 4 2
Cervical ectropion 1 1 1 1 1 1
Cervical
intraepithelial
neoplasia
2 1 2 2 2 1
See table footnotes on page 54.
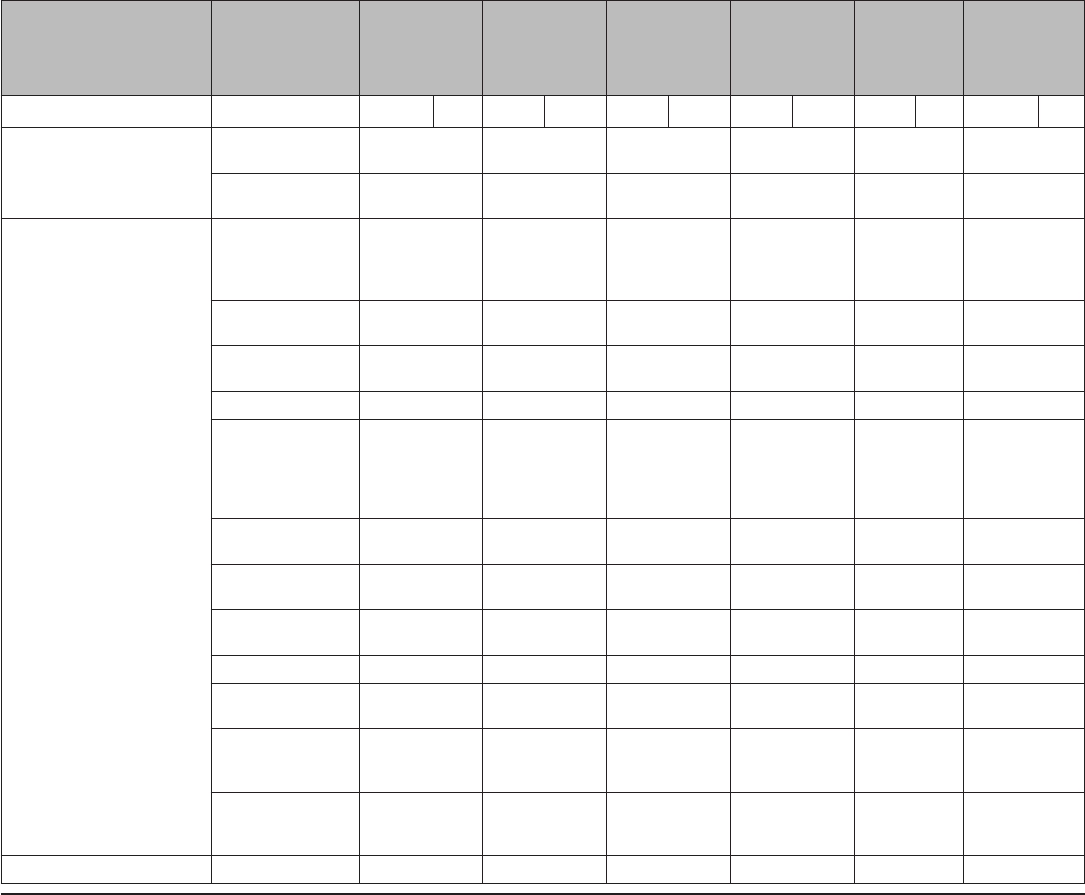
Early Release
48 MMWR / June 14, 2013 / Vol. 62
Appendix A (Continued)
Summary Chart of U.S. Medical Eligibility Criteria for Contraceptive Use, 2010
Condition Sub-condition
Combined
pill, patch,
ring
Progestin-
only pill Injection Implant LNG-IUD Copper-IUD
I C I C I C I C I C I C
Cirrhosis a) Mild
(compensated)
1 1 1 1 1 1
b) Severe
†
(decompensated)
4 3 3 3 3 1
DVT/PE a) History of
DVT/PE, not
on anticoagulant
therapy
i) higher risk for
recurrent DVT/PE
4 2 2 2 2 1
ii) lower risk for
recurrent DVT/PE
3 2 2 2 2 1
b) Acute DVT/PE 4 2 2 2 2 2
c) DVT/PE and
established on
anticoagulant
therapy for at least 3
months
i) higher risk for
recurrent DVT/PE
4* 2 2 2 2 2
ii) lower risk for
recurrent DVT/PE
3* 2 2 2 2 2
d) Family history
(rst-degree relatives)
2 1 1 1 1 1
e) Major surgery
(i) with prolonged
immobilization
4 2 2 2 2 1
(ii) without
prolonged
immobilization
2 1 1 1 1 1
f) Minor
surgery without
immobilization
1 1 1 1 1 1
Depressive disorders 1* 1* 1* 1* 1* 1*
See table footnotes on page 54.

Early Release
MMWR / June 14, 2013 / Vol. 62 49
Appendix A (Continued)
Summary Chart of U.S. Medical Eligibility Criteria for Contraceptive Use, 2010
Condition Sub-condition
Combined
pill, patch,
ring
Progestin-
only pill Injection Implant LNG-IUD Copper-IUD
I C I C I C I C I C I C
Diabetes mellitus a) History of
gestational diabetes
mellitus only
1 1 1 1 1 1
b) Non-vascular
disease
(i) non-insulin
dependent
2 2 2 2 2 1
(ii) insulin
dependent
†
2 2 2 2 2 1
c) Nephropathy/
retinopathy/
neuropathy
†
3/4* 2 3 2 2 1
d) Other vascular
disease or diabetes of
>20 years’ duration
†
3/4* 2 3 2 2 1
Endometrial cancer
†
1 1 1 1 4 2 4 2
Endometrial
hyperplasia
1 1 1 1 1 1
Endometriosis 1 1 1 1 1 2
Epilepsy
†
(see also Drug
Interactions)
1* 1* 1* 1* 1
1
Gallbladder disease a) Symptomatic
(i) treated by
cholecystectomy
2 2 2 2 2 1
(ii) medically
treated
3 2 2 2 2 1
(iii) current 3 2 2 2 2 1
b) Asymptomatic
2 2 2 2 2 1
Gestational
trophoblastic disease
a) Decreasing or
undetectable ß-hCG
levels
1 1 1 1 3 3
b) Persistently
elevated ß-hCG
levels or malignant
disease
†
1 1 1 1 4 4
Headaches a) Non-migrainous 1* 2* 1* 1* 1* 1* 1* 1* 1* 1* 1*
b) Migraine
i) without aura,
age <35
2* 3* 1* 2* 2* 2* 2* 2* 2* 2* 1*
ii) without aura,
age >35
3* 4* 1* 2* 2* 2* 2* 2* 2* 2* 1*
iii) with aura, any
age
4* 4* 2* 3* 2* 3* 2* 3* 2* 3* 1*
See table footnotes on page 54.
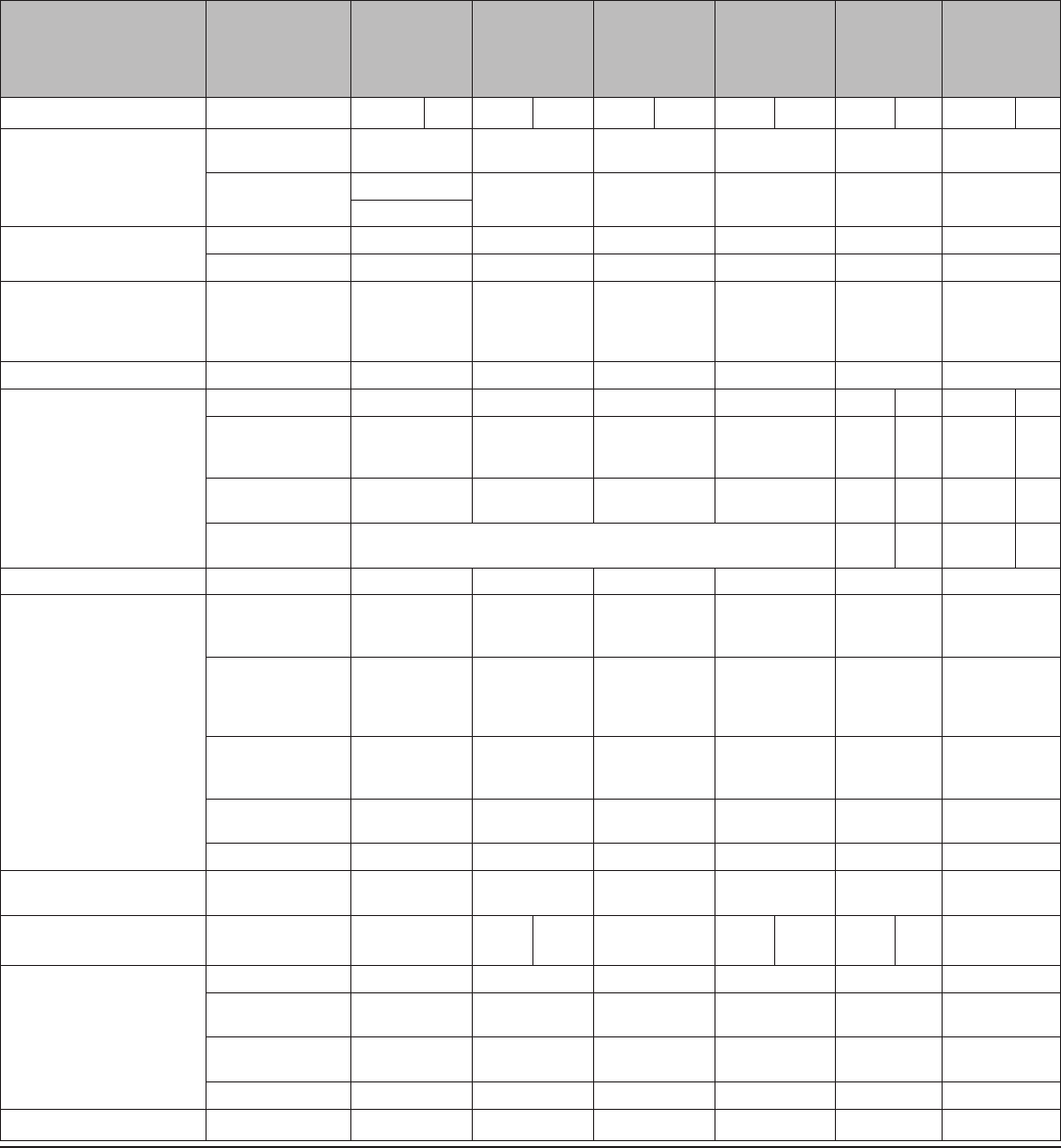
Early Release
50 MMWR / June 14, 2013 / Vol. 62
Appendix A (Continued)
Summary Chart of U.S. Medical Eligibility Criteria for Contraceptive Use, 2010
Condition Sub-condition
Combined
pill, patch,
ring
Progestin-
only pill Injection Implant LNG-IUD Copper-IUD
I C I C I C I C I C I C
History of bariatric surgery
†
a) Restrictive
procedures
1 1 1 1 1 1
b) Malabsorptive
procedures
COCs: 3 3 1 1 1 1
P/R: 1
History of cholestasis a) Pregnancy-related 2 1 1 1 1 1
b) Past COC-related 3 2 2 2 2 1
History of high blood pressure
during pregnancy
2 1 1 1 1 1
History of pelvic surgery 1 1 1 1 1 1
HIV High risk 1 1 1* 1 2 2 2 2
HIV infected
(see also Drug
Interactions)
†
1* 1* 1* 1* 2 2 2 2
AIDS (see also Drug
Interactions)
†
1* 1* 1* 1* 3 2* 3 2*
Clinically well on
therapy
If on treatment, see Drug Interactions 2 2 2 2
Hyperlipidemias 2/3* 2* 2* 2* 2* 1*
Hypertension a) Adequately
controlled
hypertension
3* 1* 2* 1* 1 1
b) Elevated blood
pressure levels
(properly taken
measurements)
(i) systolic 140-
159 or diastolic
90-99
3 1 2 1 1 1
(ii) systolic ≥160
or diastolic ≥100
†
4 2 3 2 2 1
c) Vascular disease 4 2 3 2 2 1
Inammatory bowel disease (Ulcerative colitis,
Crohn’s disease)
2/3* 2 2 1 1 1
Ischemic heart
disease
†
Current and
history of
4 2 3 3 2 3 2 3 1
Liver tumors a) Benign
i) Focal nodular
hyperplasia
2 2 2 2 2 1
ii) Hepatocellular
adenoma
†
4 3 3 3 3 1
b) Malignant
†
4 3 3 3 3 1
Malaria 1 1 1 1 1 1
See table footnotes on page 54.
Early Release
MMWR / June 14, 2013 / Vol. 62 51
Appendix A (Continued)
Summary Chart of U.S. Medical Eligibility Criteria for Contraceptive Use, 2010
Condition Sub-condition
Combined
pill, patch,
ring
Progestin-
only pill Injection Implant LNG-IUD Copper-IUD
I C I C I C I C I C I C
Multiple risk factors for arterial
cardiovascular disease
(such as older age,
smoking, diabetes
and hypertension)
3/4* 2* 3* 2* 2 1
Obesity
a) >30 kg/m
2
BMI 2 1 1 1 1 1
b) Menarche to
<18 years and
>30 kg/m
2
BMI
2 1 2 1 1 1
Ovarian cancer
†
1 1 1 1 1 1
Parity a) Nulliparous 1 1 1 1 2 2
b) Parous 1 1 1 1 1 1
Past ectopic
pregnancy
1 2 1 1 1 1
Pelvic inammatory disease a) Past, (assuming no
current risk factors
of STIs)
(i) with
subsequent
pregnancy
1 1 1 1 1 1 1 1
(ii) without
subsequent
pregnancy
1 1 1 1 2 2 2 2
b) Current 1 1 1 1 4 2* 4 2*
Peripartum cardiomyopathy
†
a) Normal or mildly
impaired cardiac
function
(i) <6 months 4 1 1 1 2 2
(ii) >6 months 3 1 1 1 2 2
b) Moderately or
severely impaired
cardiac function
4 2 2 2 2 2
Postabortion a) First trimester 1* 1* 1* 1* 1* 1*
b) Second trimester 1* 1* 1* 1* 2 2
c) Immediately post-
septic abortion
1* 1* 1* 1* 4 4
Postpartum
(see also Breastfeeding)
a) <21 days 4 1 1 1
b) 21 days to
42 days
(i) with other risk
factors for VTE 3* 1 1 1
(ii) without other
risk factors for
VTE
2 1 1 1
c) >42 days 1 1 1 1
See table footnotes on page 54.
Early Release
52 MMWR / June 14, 2013 / Vol. 62
Appendix A (Continued)
Summary Chart of U.S. Medical Eligibility Criteria for Contraceptive Use, 2010
Condition Sub-condition
Combined
pill, patch,
ring
Progestin-
only pill Injection Implant LNG-IUD Copper-IUD
I C I C I C I C I C I C
Postpartum (in breastfeeding
or non-breastfeeding women,
including post-cesarean
section)
a) <10 minutes after
delivery of the
placenta
2 1
b) 10 minutes after
delivery of the
placenta to
< 4 weeks
2 2
c) >4 weeks 1 1
d) Puerperal sepsis 4 4
Pregnancy NA* NA* NA* NA* 4* 4*
Rheumatoid
arthritis
a) On
immunosuppressive
therapy
2 1 2/3* 1 2 1 2 1
b) Not on
immunosuppressive
therapy
2 1 2 1 1 1
Schistosomiasis a) Uncomplicated 1 1 1 1 1 1
b) Fibrosis of the
liver
†
1 1 1 1 1 1
Severe dysmenorrhea 1 1 1 1 1 2
STIs a) Current purulent
cervicitis or
chlamydial infection
or gonorrhea
1 1 1 1 4 2* 4 2*
b) Other STIs
(excluding HIV and
hepatitis)
1 1 1 1 2 2 2 2
c) Vaginitis
(including
trichomonas
vaginalis and
bacterial vaginosis)
1 1 1 1 2 2 2 2
d) Increased risk of
STIs
1 1 1 1
2/3*
2
2/3*
2
Smoking a) Age <35 2 1 1 1 1 1
b) Age >35, <15
cigarettes/day
3 1 1 1 1 1
c) Age >35, >15
cigarettes/day
4 1 1 1 1 1
Solid organ
transplantation
†
a) Complicated 4 2 2 2 3 2 3 2
b) Uncomplicated 2* 2 2 2 2 2
Stroke
†
History of
cerebrovascular
accident
4 2 3 3 2 3 2 1
See table footnotes on page 54.
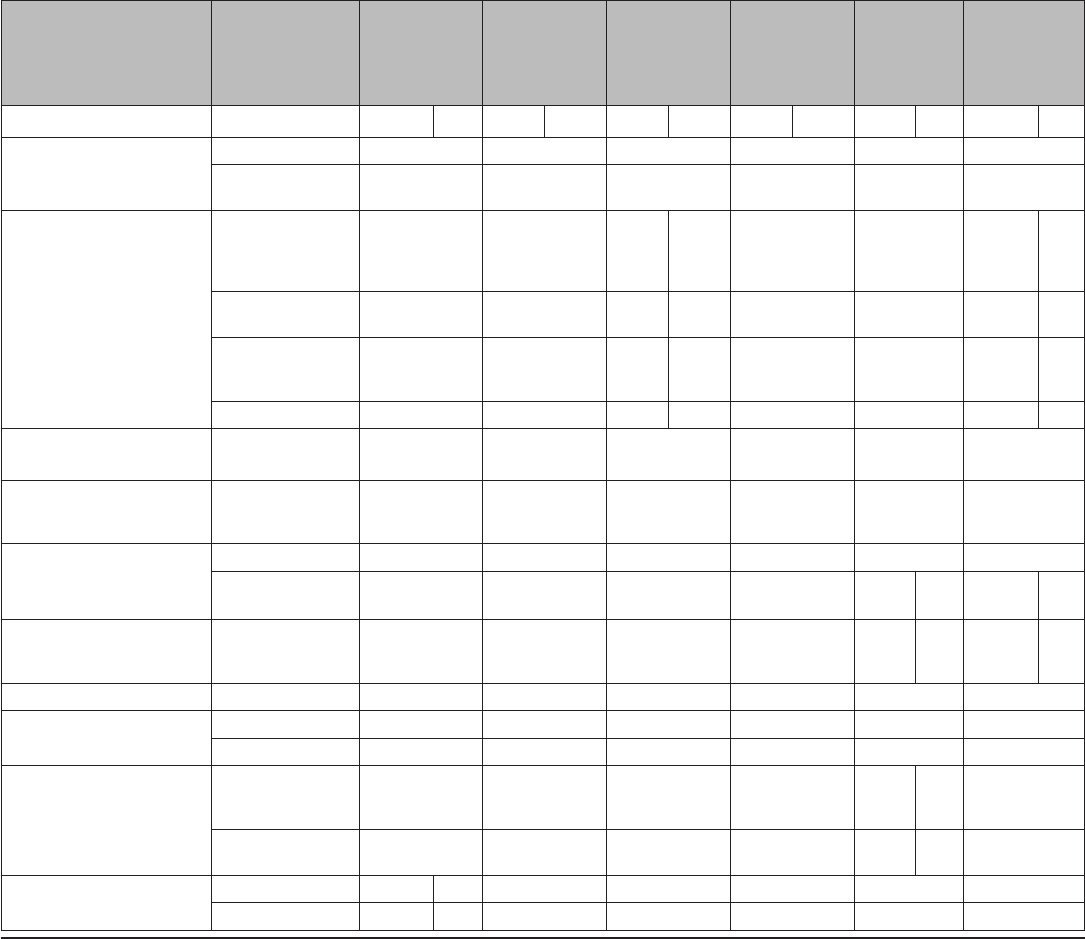
Early Release
MMWR / June 14, 2013 / Vol. 62 53
Appendix A (Continued)
Summary Chart of U.S. Medical Eligibility Criteria for Contraceptive Use, 2010
Condition Sub-condition
Combined
pill, patch,
ring
Progestin-
only pill Injection Implant LNG-IUD Copper-IUD
I C I C I C I C I C I C
Supercial venous thrombosis a) Varicose veins 1 1 1 1 1 1
b) Supercial
thrombophlebitis
2 1 1 1 1 1
Systemic lupus
erythematosus
†
a) Positive (or
unknown)
antiphospholipid
antibodies
4 3 3 3 3 3 1 1
b) Severe
thrombocytopenia
2 2 3 2 2 2* 3* 2*
c)
Immunosuppressive
treatment
2 2 2 2 2 2 2 1
d) None of the above 2 2 2 2 2 2 1 1
rombogenic
mutations
†
4* 2* 2* 2* 2* 1*
yroid disorders Simple goiter/
hyperthyroid/
hypothyroid
1 1 1 1 1 1
Tuberculosis
†
(see also Drug
Interactions)
a) Non-pelvic 1* 1* 1* 1* 1 1
b) Pelvic 1* 1* 1* 1* 4 3 4 3
Unexplained vaginal bleeding (suspicious for
serious condition)
before evaluation
2* 2*
3* 3* 4* 2* 4* 2*
Uterine broids 1 1 1 1 2 2
Valvular heart
disease
a) Uncomplicated 2 1 1 1 1 1
b) Complicated
†
4 1 1 1 1 1
Vaginal bleeding
patterns
a) Irregular pattern
without heavy
bleeding
1 2 2 2 1 1 1
b) Heavy or
prolonged bleeding
1* 2* 2* 2* 1* 2* 2*
Viral hepatitis a) Acute or are 3/4* 2 1 1 1 1 1
b) Carrier/Chronic 1 1 1 1 1 1 1
See table footnotes on page 54.
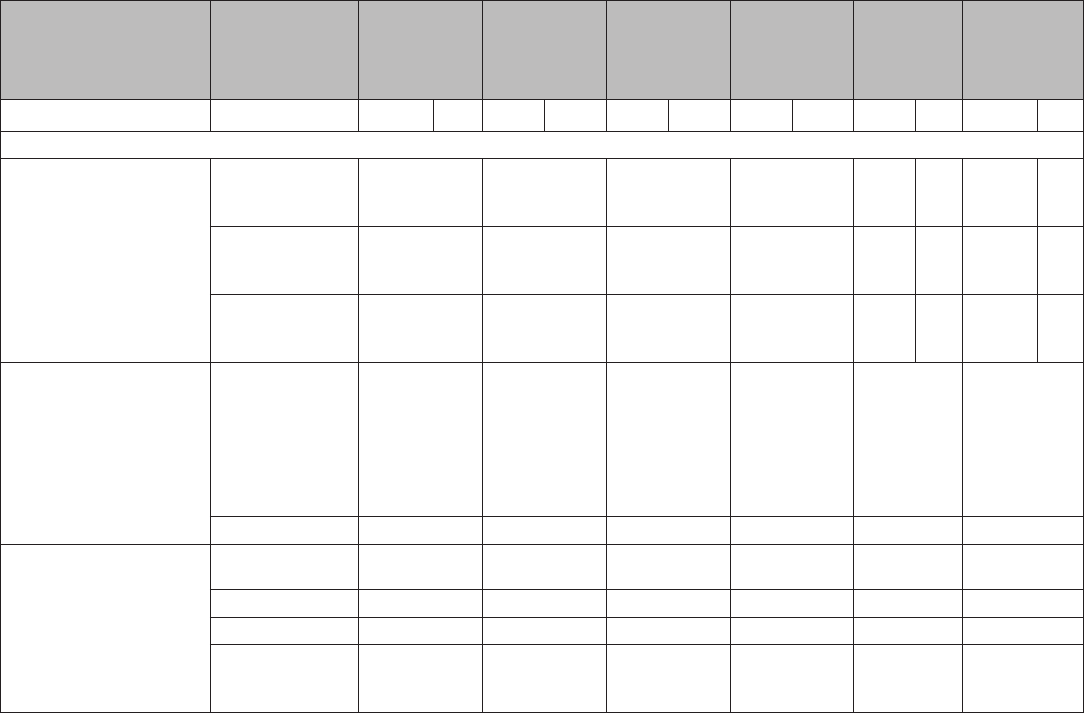
Early Release
54 MMWR / June 14, 2013 / Vol. 62
Condition Sub-condition
Combined
pill, patch,
ring
Progestin-
only pill Injection Implant LNG-IUD Copper-IUD
I C I C I C I C I C I C
Drug Interactions
Antiretroviral therapy a) Nucleoside
reverse transcriptase
inhibitors
1* 1 1 1 2/3* 2* 2/3* 2*
b) Non-nucleoside
reverse transcriptase
inhibitors
2* 2* 1 2* 2/3* 2* 2/3* 2*
c) Ritonavir-boosted
protease inhibitors
3* 3* 1 2* 2/3* 2* 2/3* 2*
Anticonvulsant therapy a) Certain
anticonvulsants
(phenytoin,
carbamazepine,
barbiturates,
primidone,
topiramate,
oxcarbazepine)
3* 3* 1 2* 1 1
b) Lamotrigine 3* 1 1 1 1 1
Antimicrobial therapy a) Broad spectrum
antibiotics
1 1 1 1 1 1
b) Antifungals 1 1 1 1 1 1
c) Antiparasitics 1 1 1 1 1 1
d) Rifampicin or
rifabutin therapy
3* 3*
1
2* 1 1
Abbreviations: AIDS = acquired immunodeficiency syndrome; BMI = body mass index; C = continuation of contraceptive method; COC = combined oral
contraceptive; Cu-IUD = copper-containing intrauterine device; DVT = deep venous thrombosis; hCG = human chorionic gonadotropin; HIV = human
immunodeficiency virus; I = initiation of contraceptive method; LNG-IUD = levonorgestrel-releasing intrauterine device; NA = not applicable;
PE = pulmonary embolism; STI = sexually transmitted infection; VTE = venous thromboembolism.
Source: Modified from CDC. Summary chart of U.S. medical eligibility criteria for contraceptive use. Atlanta, GA: CDC; 2012. (Available at http://www.
cdc.gov/reproductivehealth/UnintendedPregnancy/USMEC.htm.)
* Please see the complete guidance for a clarification to this classification: www.cdc.gov/reproductivehealth/unintendedpregnancy/USMEC.htm.
†
Condition that exposes a woman to increased risk as a result of unintended pregnancy.
Appendix A (Continued)
Summary Chart of U.S. Medical Eligibility Criteria for Contraceptive Use, 2010

Early Release
MMWR / June 14, 2013 / Vol. 62 55
Contraceptive method
When to start (if the provider is
reasonably certain that the woman is
not pregnant)
Additional contraception
(i.e., back-up) needed
Examinations or tests needed
before initiation*
Copper-containing IUD Anytime Not needed Bimanual examination and cervical
inspection
†
Levonorgestrel-releasing IUD Anytime If >7 days after menses started, use
back-up method or abstain for 7 days.
Bimanual examination and cervical
inspection
†
Implant Anytime If >5 days after menses started, use
back-up method or abstain for 7 days.
None
Injectable Anytime If >7 days after menses started, use
back-up method or abstain for 7 days.
None
Combined hormonal contraceptive Anytime If >5 days after menses started, use
back-up method or abstain for 7 days.
Blood pressure measurement
Progestin-only pill Anytime If >5 days after menses started, use
back-up method or abstain for 2 days.
None
Abbreviations: BMI = body mass index; HIV=human immunodeficiency virus; IUD=intrauterine device; STD=sexually transmitted disease; U.S. MEC=U.S. Medical
Eligibility Criteria for Contraceptive Use, 2010.
* Weight (BMI) measurement is not needed to determine medical eligibility for any methods of contraception because all methods can be used (U.S. MEC 1) or generally
can be used (U.S. MEC 2) among obese women (Box 2). However, measuring weight and calculating BMI (weight [kg]/height [m]
2
) at baseline might be helpful for
monitoring any changes and counseling women who might be concerned about weight change perceived to be associated with their contraceptive method.
†
Most women do not require additional STD screening at the time of IUD insertion if they have already been screened according to CDC’s STD Treatment Guidelines
(available at http://www.cdc.gov/std/treatment). If a woman has not been screened according to guidelines, screening can be performed at the time of IUD insertion,
and insertion should not be delayed. Women with purulent cervicitis or current chlamydial infection or gonorrhea should not undergo IUD insertion (U.S. MEC 4).
Women who have a very high individual likelihood of STD exposure (e.g., those with a currently infected partner) generally should not undergo IUD insertion
(U.S. MEC 3) (Box 2). For these women, IUD insertion should be delayed until appropriate testing and treatment occurs.
Appendix B
When To Start Using Specific Contraceptive Methods

Early Release
56 MMWR / June 14, 2013 / Vol. 62
TABLE. Examinations and tests needed before initiation of contraceptive methods
Examination or test
Contraceptive method and class
Cu-IUD and
LNG-IUD Implant Injectable CHC POP Condom
Diaphragm or
cervical cap Spermicide
Examination
Blood pressure C C C A* C C C C
Weight (BMI) (weight [kg]/
height [m]
2
)
—
†
—
†
—
†
—
†
—
†
C C C
Clinical breast examination C C C C C C C C
Bimanual examination and
cervical inspection
A C C C C C A
§
C
Laboratory test
Glucose C C C C C C C C
Lipids C C C C C C C C
Liver enzymes C C C C C C C C
Hemoglobin C C C C C C C C
Thrombogenic mutations C C C C C C C C
Cervical cytology
(Papanicolaou smear)
C C C C C C C C
STD screening with laboratory
tests
—
¶
C C C C C C C
HIV screening with laboratory
tests
C C C C C C C C
Abbreviations: BMI = body mass index; CHC = combined hormonal contraceptive; Cu-IUD = copper-containing intrauterine device; DMPA = depot medroxyprogesterone
acetate; HIV = human immunodeficiency virus; LNG-IUD = levonorgestrel-releasing intrauterine device; POP = progestin-only pill; STD = sexually transmitted disease;
U.S. MEC = U.S. Medical Eligibility Criteria for Contraceptive Use, 2010.
* In cases in which access to health care might be limited, the blood pressure measurement can be obtained by the woman in a nonclinical setting (e.g., pharmacy
or fire station) and self-reported to the provider.
†
Weight (BMI) measurement is not needed to determine medical eligibility for any methods of contraception because all methods can be used (U.S. MEC 1) or generally
can be used (U.S. MEC 2) among obese women (Box 2). However, measuring weight and calculating BMI at baseline might be helpful for monitoring any changes
and counseling women who might be concerned about weight change perceived to be associated with their contraceptive method.
§
A bimanual examination (not cervical inspection) is needed for diaphragm fitting.
¶
Most women do not require additional STD screening at the time of IUD insertion if they have already been screened according to CDC’s STD Treatment Guidelines
(available at http://www.cdc.gov/std/treatment). If a woman has not been screened according to guidelines, screening can be performed at the time of IUD insertion
and insertion should not be delayed. Women with purulent cervicitis or current chlamydial infection or gonorrhea should not undergo IUD insertion (U.S. MEC 4).
Women who have a very high individual likelihood of STD exposure (e.g., those with a currently infected partner) generally should not undergo IUD insertion
(U.S. MEC 3). For these women, IUD insertion should be delayed until appropriate testing and treatment occurs.
The examinations or tests noted apply to women who are
presumed to be healthy. Those with known medical problems
or other special conditions might need additional examinations
or tests before being determined to be appropriate candidates
for a particular method of contraception. The U.S. Medical
Eligibility Criteria for Contraceptive Use, 2010 (U.S. MEC),
might be useful in such circumstances (5). The following
classification was considered useful in differentiating the
applicability of the various examinations or tests:
Class A: essential and mandatory in all circumstances for
safe and effective use of the contraceptive method.
Class B: contributes substantially to safe and effective use,
but implementation may be considered within the public
health and/or service context; risk of not performing an
examination or test should be balanced against the benefits
of making the contraceptive method available.
Appendix C
Examinations and Tests Needed Before Initiation of Contraceptive Methods
Class C: does not contribute substantially to safe and
effective use of the contraceptive method.
These classifications focus on the relationship of the
examinations or tests to safe initiation of a contraceptive
method. They are not intended to address the appropriateness
of these examinations or tests in other circumstances. For
example, some of the examinations or tests that are not deemed
necessary for safe and effective contraceptive use might be
appropriate for good preventive health care or for diagnosing
or assessing suspected medical conditions.
No examinations or tests are needed before initiating
condoms or spermicides. A bimanual examination is necessary
for diaphragm fitting. A bimanual examination and cervical
inspection are needed for cervical cap fitting.

Early Release
MMWR / June 14, 2013 / Vol. 62 57
TABLE. Routine follow-up after contraceptive initiation
Action
Contraceptive method
Cu-IUD or LNG-IUD Implant Injectable CHC POP
General follow-up
Advise women to return at any time to discuss side effects or other
problems or if they want to change the method. Advise women
using IUDs, implants, or injectables when the IUD or implant
needs to be removed or when a reinjection is needed. No routine
follow-up visit is required.
X X X X X
Other routine visits
Assess the woman’s satisfaction with her current method and
whether she has any concerns about method use.
X X X X X
Assess any changes in health status, including medications, that
would change the method’s appropriateness for safe and
effective continued use based on U.S. MEC (i.e., category 3 and 4
conditions and characteristics) (Box 2).
X X X X X
Consider performing an examination to check for the presence of
IUD strings.
X — — — —
Consider assessing weight changes and counseling women who
are concerned about weight change perceived to be associated
with their contraceptive method.
X X X X X
Measure blood pressure. — — — X —
Abbreviations: CHC = combined hormonal contraceptive; Cu-IUD = copper-containing intrauterine device; HIV = human immunodeficiency virus; IUD = intrauterine
device; LNG-IUD = levonorgestrel-releasing intrauterine device; POP = progestin-only pill; U.S. MEC = U.S. Medical Eligibility Criteria for Contraceptive Use, 2010.
Appendix D
Routine Follow-Up After Contraceptive Initiation
These recommendations address when routine follow-up
is recommended for safe and effective continued use of
contraception for healthy women. The recommendations refer
to general situations and might vary for different users and
different situations. Specific populations that might benefit
from more frequent follow-up visits include adolescents, those
with certain medical conditions or characteristics, and those
with multiple medical conditions.
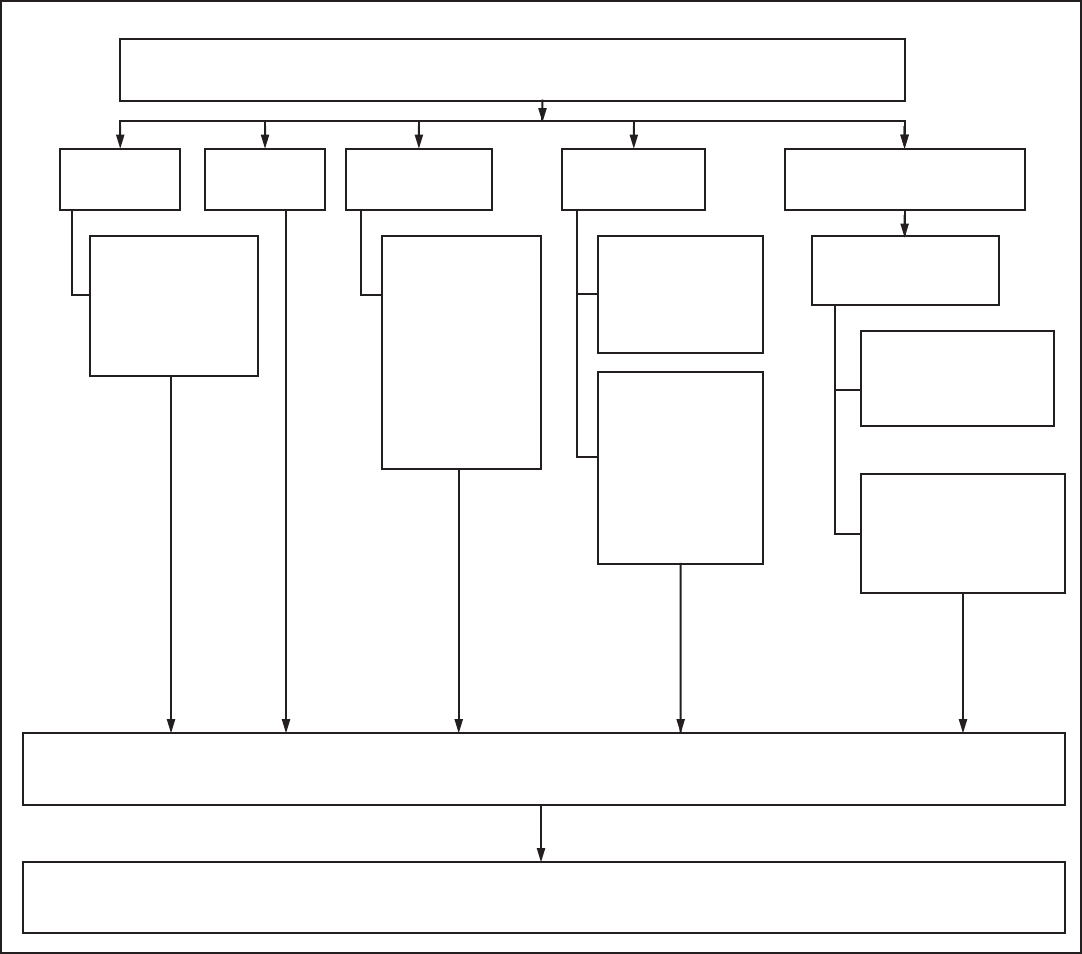
Early Release
58 MMWR / June 14, 2013 / Vol. 62
Appendix E
Management of Women with Bleeding Irregularities While Using Contraception
If bleeding persists, or if the woman requests it, medical treatment can be considered.*
Cu-IUD
users
For unscheduled
spotting or light
bleeding or for heavy
or prolonged bleeding:
•
NSAIDs (5–7 days
of treatment)
LNG-IUD
users
†
Implant
users
†
For unscheduled
spotting or light
bleeding or heavy/
prolonged bleeding:
•
NSAIDs (5–7 days
of treatment)
•
Hormonal treatment
(if medically eligible)
with COCs or
estrogen (10–20 days
of treatment)
Injectable
(DMPA) users
For unscheduled
spotting or light
bleeding:
•
NSAIDs (5–7 days
of treatment)
For heavy or
prolonged bleeding:
•
NSAIDs (5–7 days of
treatment)
•
Hormonal treatment
(if medically eligible)
with COCs or estrogen
(10–20 days of
treatment)
CHC users (extended or
continuous regimen)
Hormone-free interval
for 3–4 consecutive days
Not recommended during
the rst 21 days of
extended or continuous
CHC use
Not recommended more
than once per month
because contraceptive
eectiveness might be
reduced
If bleeding disorder persists or woman nds it unacceptable
Counsel on alternative methods and oer another method, if desired.
Abbreviations: CHC = combined hormonal contraceptive; COC = combined oral contraceptive; Cu-IUD = copper-containing intrauterine device; DMPA = depot
medroxyprogesterone acetate; LNG-IUD = levonorgestrel-releasing intrauterine device; NSAIDs = nonsteroidal antiinflammatory drugs.
* If clinically warranted, evaluate for underlying condition. Treat the condition or refer for care.
†
Heavy or prolonged bleeding, either unscheduled or menstrual, is uncommon.
Early Release
MMWR / June 14, 2013 / Vol. 62 59
Appendix F
Management of the IUD when a Cu-IUD or an LNG-IUD User Is Found To Have
Pelvic Inflammatory Disease
• Treat PID.*
• Counsel about condom use.
• IUD does not need to be removed.
Woman wants to continue IUD. Woman wants to discontinue IUD.
Clinical improvement No clinical improvement
• Offer another contraceptive method.
• Offer emergency contraception.
Continue IUD.
Reassess in 24–48 hours. Remove IUD after beginning antibiotics.
• Continue antibiotics.
• Consider removal of IUD.
• Offer another contraceptive method.
• Offer emergency contraception.
Abbreviations: Cu-IUD = copper-containing IUD; IUD = intrauterine device; LNG-IUD = levonorgestrel-releasing IUD; PID = pelvic inflammatory disease.
* Treat according to CDC’s STD Treatment Guidelines (available at http://www.cdc.gov/std/treatment).
Early Release
60 MMWR / June 14, 2013 / Vol. 62
U.S. Selected Practice Recommendations for Contraceptive Use Participants
CDC Steering Committee
Kathryn M. Curtis, PhD (Chair), Denise J. Jamieson, MD, Polly A. Marchbanks, PhD, Naomi K. Tepper, MD, CDC, Atlanta, Georgia.
Invited Meeting Participants,
October 21–22, 2010, Atlanta, Georgia
Herbert B. Peterson, MD, University of North Carolina, Chapel Hill, North Carolina (Chair); Abbey Berenson, MD, University of Texas Medical Branch,
Nassau Bay, Texas; Philip Darney, MD, University of California, San Francisco, California; David Eisenberg, MD, Washington University, St. Louis,
Missouri; Paula Hillard, MD, Stanford University, Palo Alto, California; Andrew Kaunitz, MD, University of Florida, Jacksonville, Florida; Trent MacKay,
MD, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland; Mary Mitchell, American College
of Obstetricians and Gynecologists, Washington, DC; Michael Policar, MD, University of California, San Francisco, California; Sarah Prager, University of
Washington, Seattle, Washington.
Systematic Review Presenters and Authors
Meeting, October 4–7, 2011, Atlanta, Georgia
Kathryn M. Curtis, PhD, Suzanne G. Folger, PhD, Emily M. Godfrey, MD, Denise J. Jamieson, MD, Gary Jeng, PhD, Polly A. Marchbanks, PhD, Sarah
Murtaza, MPH, Maria W. Steenland, MPH, Naomi K. Tepper, MD, Crystal P. Tyler, PhD, Maura K. Whiteman, PhD, Lauren B. Zapata, PhD, CDC, Atlanta,
Georgia; Dalia Brahmi, MD, Ipas, Carrboro, NC; Nathalie Kapp, MD, Mary Lyn E. Gaffield, PhD, Maria I. Rodriguez, MD, World Health Organization,
Geneva, Switzerland; Tara P. Cleary, MD, Carrie Cwiak, MD, Emory University, Atlanta, Georgia; Jennifer Salcedo, MD, University of California, Los Angeles,
California; Ira Sharlip, MD, American Urological Association and University of California, San Francisco, California.
Invited Meeting Participants,
October 4–7, 2011, Atlanta, Georgia
Willard Cates, Jr., MD, FHI360, Research Triangle Park, North Carolina (Chair); Herbert B. Peterson, MD, University of North Carolina, Chapel Hill, North
Carolina (Chair); Rebecca Allen, MD, American Society for Reproductive Medicine and Brown University, Providence, Rhode Island; Abbey Berenson, MD,
University of Texas Medical Branch, Nassau Bay, Texas; Paul Blumenthal, MD, Stanford University, Palo Alto, California; Mitchell Creinin, MD, University
ofCalifornia,Davis,California;VanessaCullins,MD,PlannedParenthoodFederationofAmerica,NewYork,NewYork;JenniferDietrich,MD,Baylor
College of Medicine, Houston, Texas; Linda Dominguez, Southwest Women’s Health, Albuquerque, New Mexico; Alison Edelman, MD, Oregon Health
Sciences University, Portland, Oregon; David Eisenberg, MD, Washington University, St. Louis, Missouri; Melissa Gilliam, MD, The University of Chicago,
Chicago,Illinois;MarjiGold,MD,AlbertEinsteinCollegeof Medicine,Bronx,NewYork;AlisaGoldberg,MD,BrighamandWomen’sHospitaland
Planned Parenthood of Massachusetts, Boston, Massachusetts; Robert Hatcher, MD, Emory University, Atlanta, Georgia; Mark Hathaway, MD, Association
for Reproductive Health Professionals and Washington Hospital Center, Washington, DC; Stephen Heartwell, DrPH, Susan Thompson Buffett Foundation,
Omaha, Nebraska; Paula Hillard, MD, Stanford University, Palo Alto, California; Andrew Kaunitz, MD, University of Florida, Jacksonville, Florida; Hal
Lawrence, MD, American College of Obstetricians and Gynecologists, Washington, DC; Laura MacIsaac, MD, Albert Einstein School of Medicine, New
York,NewYork;TrentMacKay,MD,NationalInstituteofChildHealthandHumanDevelopment,NationalInstitutesofHealth,Bethesda,Maryland;Mary
Mitchell, American College of Obstetricians and Gynecologists, Washington, DC; Susan Moskosky, MS, U.S. Department of Health and Human Services,
Rockville, Maryland; Patricia Murphy, DrPH, University of Utah, Salt Lake City, Utah; Kavita Nanda, MD, FHI360, Research Triangle Park, North Carolina;
DeborahNucatola,MD,PlannedParenthoodFederationofAmerica,NewYork,NewYork;JeffreyPeipert,MD,WashingtonUniversity,St.Louis,Missouri;
Michael Policar, MD, University of California, San Francisco, California; Sarah Prager, University of Washington, Seattle, Washington; Ira Sharlip, MD,
American Urological Association and University of California, San Francisco, California; David Soper, MD, University of South Carolina, Charleston, South
Carolina; Lisa Soule, MD, Food and Drug Administration, Silver Spring, Maryland; Maria Trent, MD, Johns Hopkins University, Baltimore, Maryland;
JamesTrussell,PhD,PrincetonUniversity,Princeton,NewJersey;CarolynWesthoff,MD,ColumbiaUniversity,NewYork,NewYork;MimiZieman,MD,
Emory University, Atlanta, Georgia.
External Reviewers
Courtney Benedict, MSN, Planned Parenthood Federation of America, Sonoma, California; Gale Burstein, MD, Erie County Department of Health, Buffalo,
NewYork;LucyChie,MD,HarvardMedicalSchool,Boston,Massachusetts;KellyCulwell,MD,UniversityofCalifornia,Davis,California;SharonSchnare,
MSN, University of Washington, Seattle, Washington.
